Dasatinib is a new, oral small-molecule tyrosine kinase inhibitor (TKI) developed by Bristol Myers Squibb for the treatment of CML. Active against all BCR-ABL mutations except T3151, it is the registered combined BCR-ABL and SRC kinase inhibitor.
When it was still in relatively early-stage development, both the US FDA and European EMEA granted accelerated approval for the use of dasatinib in adult patients with CML (all stages) that are resistant or intolerant to prior therapy, including treatment with imatinib mesylate. In addition, the drug was approved for use in the treatment of Philadelphia chromosome-positive acute lymphoblastic leukaemia in adult patients resistant or intolerant to prior therapy.
Dasatinib, which is currently marketed under the brand name Sprycel, was designated an orphan medicinal product at the end of 2005. The marketing authorisation for the EU region was obtained in November 2006.
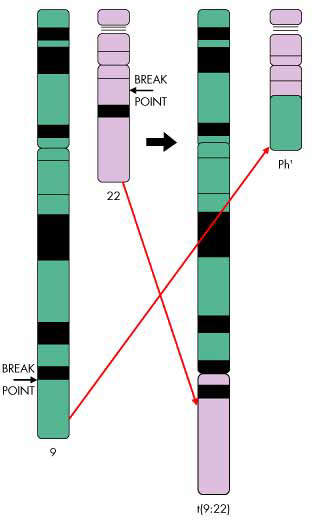
Chromosomal translocation in CML
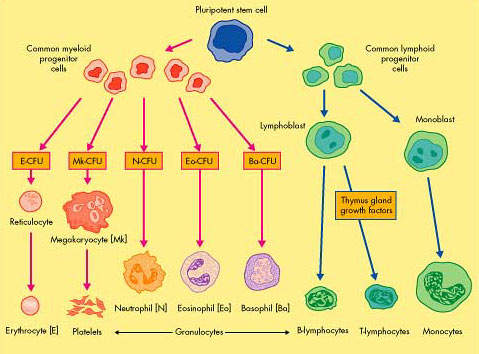
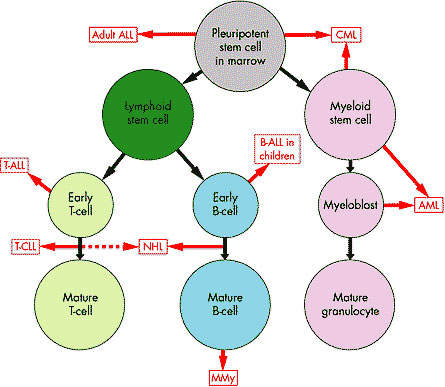
CML, one of the most common forms of leukaemia, arises from the excessive production of abnormal stem cells in the bone marrow, which eventually suppress the production of normal white blood cells.
The disease usually has three identifiable phases: the initial chronic phase, which is typically benign and lasts for an average three to five years from diagnosis, the accelerated phase and finally the blast-crisis phase.
The vast majority of patients with CML have a genetic mutation called the Philadelphia chromosome, due to reciprocal translocation between the long arms of chromosomes 9 and 22. This leads to the creation of a bcr-abl fusion gene that encodes the production of the bcr-abl protein, a tyrosine kinase that influences cell growth, differentiation and survival.
Cells containing the Philadelphia chromosome replicate rapidly producing the characteristic pattern of abnormal cells seen in the bone marrow and blood of CML patients. Because the bcr-abl fusion protein is almost never seen outside leukaemia cells, it presents an attractive therapeutic target and has been successfully exploited in the development of new treatments for CML.
Current therapies for CML
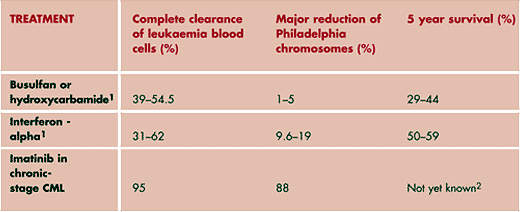
Treatment options for patients with CML include conventional cytotoxic chemotherapy, interferon-alpha, allogeneic Stem Cell Transplant (SCT), the only potentially curative therapy, and imatinib mesylate, the current gold standard.
The development of imatinib (Glivec/Gleevec), a small-molecule TKI, is the first rationally designed drug for CML. It competitively inhibits bcr-abl tyrosine kinase activity. By blocking the effects of the bcr-abl fusion protein, imatinib helps destroy leukaemic cells. It is currently indicated as a first-line treatment in patients with chronic Philadelphia-positive-chromosome CML as well as those who initially present in the accelerated or blast cell crisis phase.
While imatinib has undoubtedly had, and continues to have, a major impact in the treatment of CML, cases of imatinib-resistant CML are emerging. Combating imatinib-resistant CML is an important therapeutic challenge and one for which a new generation of TKI inhibitors, such as dasatinib, hold promise.
Dasatinib shows efficacy in imatinib-resistant CML
Although most patients with CML initially respond to treatment with imatinib, cases of imatinib resistance are increasingly being reported. Unmet clinical need therefore exists for drugs that can override imatinib resistance in patients with CML, especially in those who progress to the accelerated and blast-crisis phase.
Preclinical experience with dasatinib suggests that it possesses potent antileukaemic activity in imatinib-resistant cell lines as well as in malignant bone marrow cells isolated from patients with imatinib-resistant CML, and in mouse xenograft models of imatinib-resistant CML.
Promising antileukaemic activity has now been confirmed in an early clinical study in patients with imatinib-resistant and intolerant accelerated and blast phase CML who received twice-daily dasatinib. Patients in this ongoing phase I trial included those with no haematologic response to three-months imatinib therapy, those in whom disease had progressed despite imatinib treatment, and those who had developed nonhaematologic toxicity.
Major haematologic response, defined as the presence of less than 5% marrow blasts, were seen in 80% of patients in the accelerated phase and in 69% of patients in the blast-crisis phase of the disease. Corresponding cytogenetic response rates were 40% and 56% respectively, rates that compare very favourably with responses to imatinib. Typically about 46% of patients in the accelerated phase and 24% in the blast-crisis phase experience a major haematologic response to imatinib, while about 24% and 16% respectively have major cytogenetic responses.
The international phase II trial that also included patients with chronic-phase CML provided additional clinical data on this promising new antileukaemic drug.
The clinical trial programme also included a randomised, controlled phase-III dose optimisation trial, in addition to four single-arm phase II trials and an open-label, randomised, non-comparative phase II trial of Sprycel and high-dose imatinib.
Marketing commentary
The development of rationally designed drugs for CML represented a major breakthrough in the treatment of this disease as well as expanding treatment options for patients in all stages of the disease. Srycel (dasatinib) represents an important new addition to the TKI class of targeted therapies for CML, especially in the treatment of imatinib-resistant CML.