Delstrigo™ is a fixed-dose combination tablet of antiretroviral drugs doravirine, lamivudine, and tenofovir disoproxil fumarate indicated for the treatment of human immunodeficiency virus (HIV) 1 infection.
Merck submitted a new drug application (NDA) for Delstrigo to the US Food and Drug Administration (FDA) in October 2017. The FDA approved the drug in August 2018.
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) provided a positive opinion for the approval of the drug in September 2018. The drug was approved by the European Commission (EC) in November. Health Canada also approved Delstrigo in the same month.
HIV-1 disease symptoms and causes
HIV infection is caused by a virus that destroys CD4 T-cells. The disease weakens the immune system, making the patient susceptible to infections.
HIV can be HIV-1 or HIV-2. The former is more virulent and infective.
HIV infection can be easily transmitted through infected blood, semen or vaginal secretions. Some of the common signs of HIV infection include fever, headache, muscular and joint pain, sore throat, swollen lymph glands and rashes.
As of 2017, an estimated 36.9 million people worldwide were living with HIV.
Delstrigo mechanism of action
Delstrigo is a once-daily, fixed-dose combination tablet of non-nucleoside reverse transcriptase inhibitor (NNRTI) doravirine and nucleoside analogue reverse transcriptase inhibitors lamivudine and tenofovir disoproxil fumarate.
Doravirine is a pyridinone NNRTI that blocks HIV-1 replication by non-competitive inhibition of reverse transcriptase process in the virus. Reverse transcriptase is crucial for the growth and proliferation of the virus in the body.
Lamivudine is a synthetic nucleoside analogue that inhibits reverse transcriptase by DNA chain termination, while tenofovir disoproxil fumarate is an acyclic nucleoside phosphonate diester analogue of adenosine monophosphate that also inhibits the reverse transcriptase process.
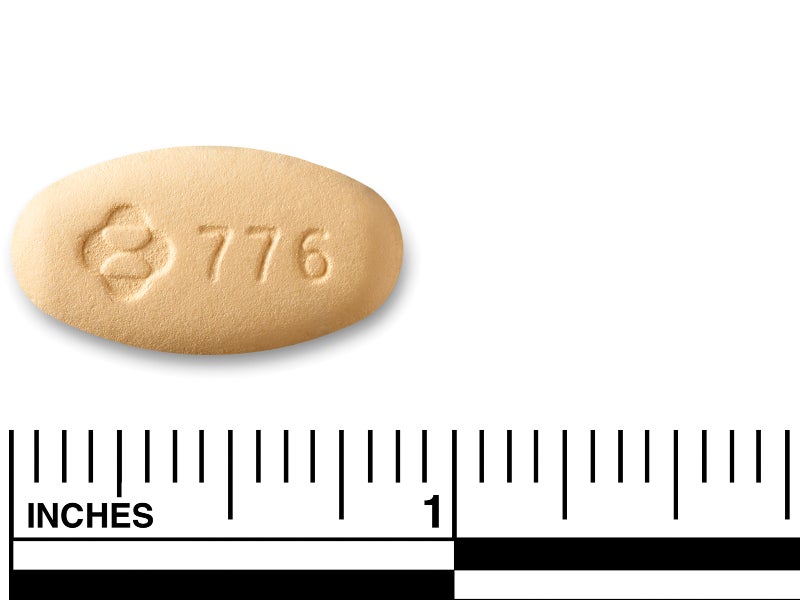
Delstrigo is available as yellow-coloured, oval, film-coated tablets, which contain 100mg of doravirine, 300mg of lamivudine and 300mg of tenofovir disoproxil fumarate.
Clinical trials on Delstrigo
The FDA’s and EC’s approvals were based on the positive outcomes of a 48-week, pivotal, randomised, multi-centre, double-blind and active-controlled Phase III clinical trial called DRIVE-AHEAD.
The trial enrolled a total of 726 patients with no prior treatment with antiretrovirals. Of these, 21% had a high viral load.
The patients were randomised either to receive Delstrigo™ or efavirenz / emtricitabine / tenofovir disoproxil fumarate (EFV/FTC/TDF). The primary endpoint of the study was non-inferior efficacy of the drug compared to EFV/FTC/TDF.
At week 48, 84% of patients treated with Delstrigo achieved viral suppression compared with 81% of those treated with EFV/FTC/TDF. Out of the patients with a high-viral load, 77% of patients treated with Delstrigo and 71% of the patients treated with EFV/FTC/TDF achieved viral suppression.
The patients treated with Delstrigo also showed superior lipid profiles compared to those on EFV/FTC/TDF and the rate of treatment discontinuation was also low for patients treated with Delstrigo.
Some of the common adverse events noticed in patients during the clinical trial were dizziness, nausea and abnormal dreams.
Marketing commentary on Merck
Merck is a US-based biopharmaceutical company known as MSD outside the US and Canada. The company is focussed on the development of novel medicines, vaccines and animal healthcare products.