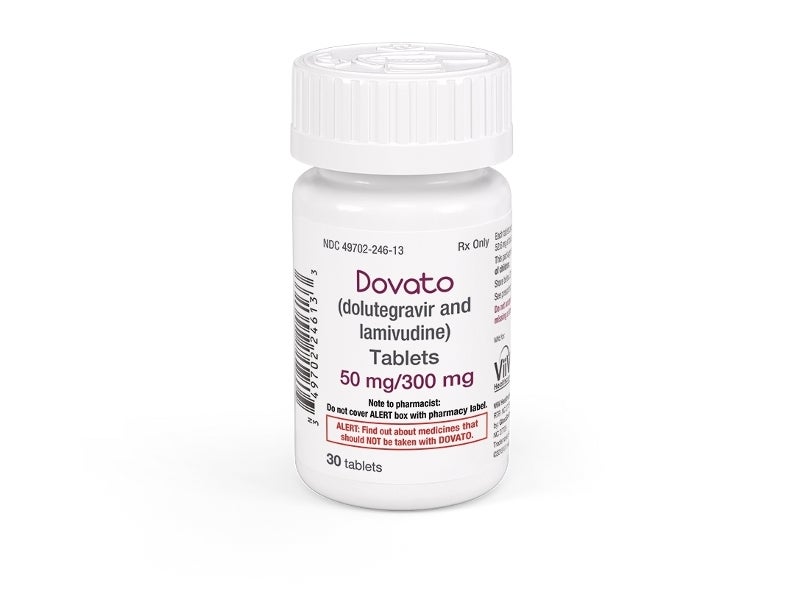
Dovato® (dolutegravir/lamivudine) is a two-drug regimen indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) in adults.
The drug was developed by ViiV Healthcare, a joint venture (JV) between GlaxoSmithKline (GSK), Pfizer and Shionogi.
A new drug application (NDA) for Dovato was submitted to the US Food and Drug Administration (FDA) in October 2018. The FDA’s approval was received in April 2019 for the treatment of HIV-1 infection in adult patients with no history of past antiretroviral (ARV) treatment.
A marketing authorisation application (MAA) was submitted to the European Medicines Agency (EMA) in September 2018, and the EMA’s Committee for Medicinal Products for Human Use (CHMP) granted a positive opinion in April 2019.
Dovato® obtained EU marketing authorisation for the treatment of HIV-1 Infection in July 2019.
The drug is also under review in South Africa, Australia, Switzerland and Canada.
HIV-1 disease causes and symptoms
HIV-1 is one of the two main types of HIV infection. It is caused by a virus that damages the immune system by destroying CD4 T-cells.
The infection can spread through infected blood and body fluids, progressing to AIDS if not treated. Fever, sore throat, muscle aches, headaches and coughing are the common symptoms of HIV infection.
An estimated 38,739 people in the US were diagnosed with HIV in 2017.
Dovato’s mechanism of action
Dovato® is a combination of 50mg of the integrase strand transfer inhibitor (INSTI) dolutegravir (Tivicay; DTG) and 300mg of the nucleoside analogue reverse transcriptase inhibitor (NRTI) lamivudine (Epivir; 3TC), which is a synthetic nucleoside analogue.
Dolutegravir binds to the active site of the integrase produced by HIV virus and blocks the strand transfer activity, which will stop the formation of integrated proviral DNA.
Lamivudine is metabolised intracellularly by phosphorylation to its active 5-triphosphate metabolite, lamivudine triphosphate (3TC-TP), which inhibits reverse transcriptase (RT) via DNA chain termination.
The drug is available in the form of a fixed-dose tablet for oral administration.
Clinical trials on Dovato®
The US FDA’s approval for Dovato was based on the positive results obtained from the Phase III GEMINI 1 and GEMINI 2 clinical trials, which together enrolled 1,433 adult HIV-1 patients with no ARV treatment history.
Dovato®was administered once daily for 148 weeks in patients with baseline HIV-1 viral loads up to 500,000 copies per millilitre during both trials.
The drug was also compared to a three-drug regimen comprising dolutegravir, two NRTIs, and tenofovir disoproxil fumarate/emtricitabine (Truvada; TDF/FTC). The primary efficacy endpoint of each trial was the achievement of plasma HIV-1 RNA rebinucleaic acid (RNA) copies to less than 50 per ml after week 48 in 91% cases.
A mean change of 0.116mg/dl was observed in fasted lipid values of patients treated with a two-drug combination of dolutegravir and lamivudine from baseline to week 48. The mean change for patients treated with a three-drug regimen was noticed as 0.154mg/dl from baseline to week 48.
Dovato® achieved a 10% non-inferior efficacy compared to the three-drug regimen during the trials and resulted in reducing exposure to the number of ARVs.
Insomnia, diarrhoea, headache, nausea, and fatigue were the most common adverse events recorded during the GEMINI I and II clinical trials.
Marketing commentary on ViiV Healthcare
Based in London, UK, ViiV Healthcare develops therapies for the treatment of HIV infection. The company was formed as a JV between GSK (85%) and Pfizer (15%) in 2009, which was later joined by Japanese pharmaceutical firm Shionogi through the acquisition of 10% of GSK’s stake in the JV in October 2012.
ViiV Healthcare currently employs 1,100 people across its operations in 20 countries. ViiV’s product portfolio of 14 antiretroviral medicines includes Dovato, Juluca®, Triumeq®, Tivicay®, Selzentry/Celsentri®, Epzicom/Kivexa®, Ziagen®, Trizivir®, Combivir®, Epivir/3TC®, Retrovir/AZT®, Lexiva/ Telzir®, Viracept® and Rescriptor®.