Rember (methylthioninium chloride) is a potential disease-modifying therapy for Alzheimer’s disease that is under development by the Singapore-based company TauRx.
The drug entered phase III pivotal clinical evaluation of LMTX in March 2010 under an open US investigational new drug initiative, following encouraging preliminary results in phase II trials.
TauRx entered into an agreement with INC Research to conduct Phase III studies on the drug for treating mild and moderate Alzheimer’s disease.
TauRx filed an application with the UK Medicines and Healthcare products Regulatory Agency in November 2009 for the approval of the drug. The company was granted a patent by European Patent Office for second-generation Rember in March 2010.
Targets for disease modification in Alzheimer’s disease
Alzheimer’s disease takes its name from Alois Alzheimer, the German physician who first described the neurodegenerative disease in 1906. Today, it is recognised as the most common form of dementia to affect those aged 65 years and older.
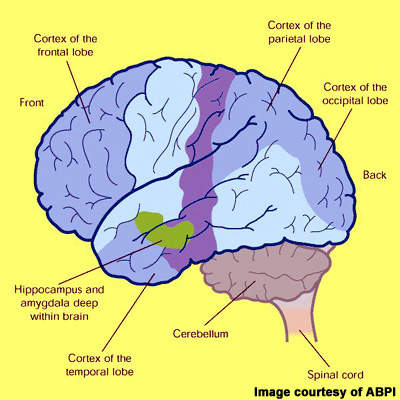
Alzheimer’s disease is characterised by progressive cognitive decline and accompanying behavioural symptoms, the effects of which are attributed to the formation of amyloid plaques and neurofibrillary tangles in the brain leading to a loss of cortical neurones and synapses.
While research into the causes of Alzheimer’s disease continues, efforts are also focusing on the development of drugs to address the underlying pathology – so-called disease-modifying treatments. Much of this effort is concentrated on the development of anti-amyloid therapies that are designed to destroy insoluble toxic beta-amyloid (b -amyloid42), aggregates that lead to the formation of amyloid plaques.
After drugs to address deficiencies in cholinergic neurotransmission, anti-amyloid therapies are the next largest group of compounds in development for Alzheimer’s disease.
However, recent failure of two anti-amyloid drugs (tarenflubril and tramiprosate) in late-stage development has placed a question mark over this approach to treatment of Alzheimer’s disease.
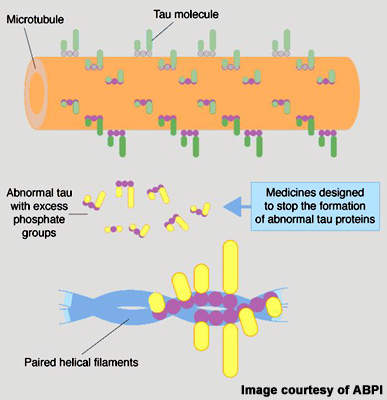
TauRx has adopted a different approach and focused instead on targeting neurofibrillary tangles. These develop in the brain when tau protein, which under normal conditions holds microtubules together, becomes hyperphosphorylated. This abnormal, insoluble form of tau protein forms aggregates that destroy neurones critical to memory. Rember, an orally administered drug, targets the tau aggregation pathway, potentially preventing the development of neurofibrillary tangles in the brain.
Promising phase II trial results
Evidence that Rember may be beneficial to patients with mild to moderate Alzheimer’s disease has emerged from a phase II trial in 321 patients. In this 50-week trial, Alzheimer’s disease patients were randomised to Rember at doses of 30, 60 or 100mg or to placebo. Assessments on the ADAS-cog scale at 24 weeks showed that patients treated with Rember experienced a 5.4 point improvement compared with no change in the placebo group (p<0.05). At 50 weeks, the improvement on ADAS-cog was 6.8 points for patients in the active treatment arm compared with a decline of 7.8 points among placebo recipients (p<0.05).
These results, which if substantiated in larger phase III trials, suggest that treatment with Rember may slow the progression of the disease in patients with mild to moderate Alzheimer’s disease as measured by changes in cognition.
Significant unmet clinical need
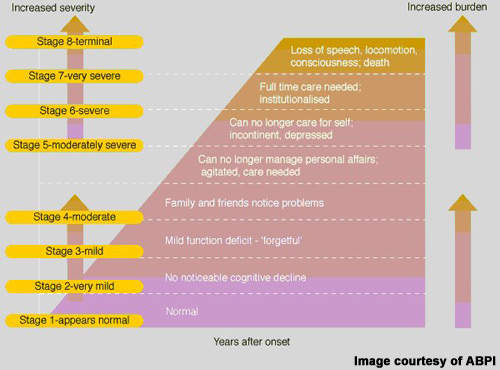
Despite some progress in treatment over the past decade, Alzheimer’s disease remains an incurable disease. Currently available anti-dementia drugs provide at best modest symptomatic improvement; their effects on cognition, executive functioning and behaviour are only temporary. None is considered to have disease-modifying effects that can stop disease progression and halt cognitive decline – the hallmark of Alzheimer’s disease. In addition, many patients fail treatment with first-line anti-dementia drugs because of poor efficacy, safety and compliance with treatment.
Because of this, a large population of Alzheimer’s disease patients exists that are eligible for treatment but for whom current anti-dementia drugs are either ineffective or of limited effectiveness. Bridging current treatment gaps is a huge technical challenge but vital to prevent the inexorable rise in prevalence of Alzheimer’s disease as the world’s population ages.
Marketing commentary
The current market for anti-dementia therapies is in its infancy with among the highest growth dynamics in the CNS market, fuelled by a growing elderly population and the need for better treatments.
While anti-amyloid therapies have been the main focus of research into disease-modifying drugs, targeting abnormal tau protein is also seen as a potentially viable strategy. Certainly, the early results with Rember are encouraging. As the phase II study investigators have highlighted, treatment with Rember achieved an 81% reduction in cognitive decline over 12 months and no significant decline in mental function was observed over 19 months.
Interestingly, imaging data indicated that the drug exerted its effect predominantly in memory-critical regions of the brain where the density of neurofibrillary tangles is greatest. Not surprisingly, outcome from the phase III trials is eagerly awaited.
Meanwhile, Wis Ta Laboratories, a subsidiary of TauRx Pharmaceuticals, signed an R&D agreement with Bayer Schering Pharma in May 2010 to jointly develop new diagnostic applications for Alzheimer’s disease.