Developed by Coughar Biotechnology, abiraterone acetate (CB7630) was discovered by the UK Institute for Cancer Research. This innovative treatment for advanced prostate cancer was subsequently acquired by BTG, who, after securing the drug’s intellectual property rights, licensed it to Coughar Biotechnology.
In July 2009, Coughar Biotechnology was acquired by Johnson & Johnson.
On 20 December 2010, Centocor Ortho Biotech, a unit of Johnson & Johnson, submitted a new drug application for abiraterone to the US Food and Drug Administration (FDA). On the same day, Janssen-Cilag International, another unit of Johnson & Johnson, filed a marketing authorisation application for Zytiga (Abiraterone acetate) to the European Medical Agency.
The drug received FDA approval in April 2011. It can be administered once daily, in combination with prednisone, to treat the metastatic castration-resistant prostate cancer (mCRPC) in men who have already received the chemotherapy containing docetaxel. The FDA approved Zytiga to treat mCRPC patients prior to chemotherapy in December 2012.
Zytiga also received a positive response from the Committee of European Medicines Agency (EMA) for Medicinal Products for Human Use (CHMP) for the expanded indication.
Prostate cancer in the UK
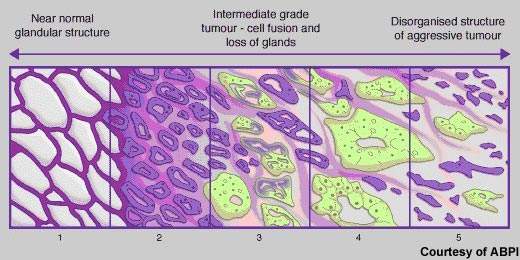
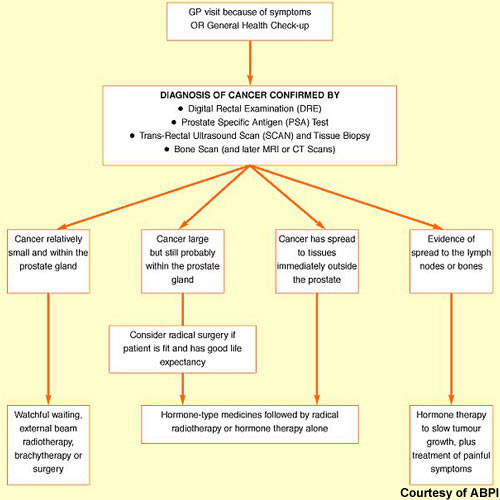
Cancer of the prostate, a gland that lies below the bladder, is the most common cancer to affect men. In the UK alone, more than 30,000 new cases occur annually and about 10,000 men die from the disease. Rare in men aged 50 years and under, the incidence of prostate cancer rises significantly with age. Most cases occur in men aged 70 years and older. Indeed, age is the biggest risk factor for prostate cancer. Risk is also increased in families with a history of the disease, especially where onset was before 60 years of age.
Although there have been major improvements in survival rates over the past 20 years, in many Western countries it remains the second most common cause of cancer-related death in men. New drugs are needed to address significant treatment gaps in advanced prostate cancer, notably in hormone refractory prostate cancer (HRPC).
Hormone therapy in prostate cancer
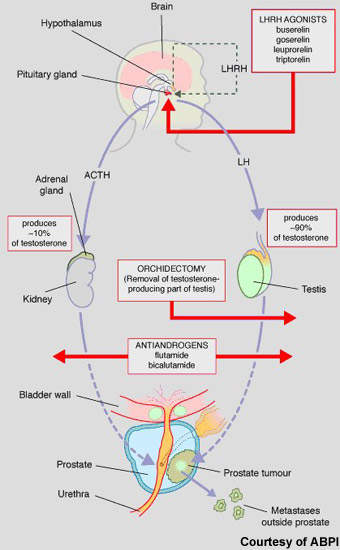
Hormone therapy has an important place in the treatment of prostate cancer, predicated on the basis that androgens (testosterone) fuel the growth of prostate cancer cells. While surgical castration is used in some cases, most patients today receive drug-based hormone therapy with either pituitary down regulators (LHRH agonists) or anti-androgens (medical castration).
Luteinising hormone releasing hormone (LHRH) agonists act via the pituitary gland to suppress testosterone production, while anti-androgens act on testosterone production in the testes. These drugs are usually administered alone but may be given concomitantly to prevent tumour flare or if the cancer is becoming resistant to single-agent therapy.
Abiraterone is a new hormonal therapy. Administered orally, it blocks cytochrome p17, a steroidal enzyme that is essential to testosterone production. By blocking the action of CYP450c17, abiraterone not only inhibits testosterone production in the testes but also in other testosterone-producing tissues such as the adrenal glands and in the tumour cells as well. In this respect it differs from currently available hormone therapies, which suppress testosterone production in the testes.
Abiraterone shows efficacy in HRPC
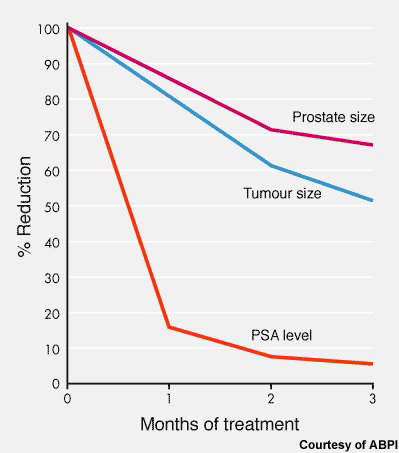
Evidence that abiraterone may be an effective hormonal treatment for prostate cancer first emerged from a phase I trial in 21 men with HRPC. In this small study, treatment with abiraterone was associated with pronounced tumour shrinkage and a fall in prostate specific antigen (PSA) levels in 70–80% of patients. These changes were also accompanied by symptomatic improvements, such as reduced pain.
Subsequent phase II trials have confirmed these encouraging early findings. In an open-label phase II study in 54 HRPC patients, treatment with abiraterone was again associated with tumour shrinkage and a fall in PSA levels.
When PSA levels subsequently rose, the addition of dexamethasone to ongoing abiraterone produced a reduction in PSA levels with about a third of patients experiencing a fall of over 50%.
A second phase II trial investigated the effects of abiraterone in men with HRPC who had also failed standard chemotherapy with docetaxel and whose PSA levels had started to rise. Treatment with abiraterone again produced a reduction in PSA levels and no evidence of tumour progression over a 12-week treatment period.
On the strength of these trials, Coughar Biotechnology initiated phase III trials in which abiraterone was evaluated in patients with metastatic HRPC who have failed prior docetaxel-based chemotherapy (trial COU-AA-301) and potentially in chemotherapy-naive patients with HRPC.
Enrolment for the Phase III trial, known as COU-AA-301, started in April 2008 and was completed in April 2009. The trial enrolled 1,195 patients across 147 hospitals in North America, Europe and Australia. Patients in the trial were randomised to treatment with abiraterone plus prednisone or prednisone plus placebo.
In November 2008, Coughar Biotechnology initiated a Phase I/II clinical trial in patients with advanced breast cancer. The trial was an open label, dose escalating trial to assess the safety and efficacy of abiraterone in UK women suffering from advanced breast cancer.
Related project
Xtandi (enzalutamide) for Treatment of Metastatic Castration-Resistant Prostate Cancer, United States of America
Xtandi (enzalutamide) is an androgen receptor inhibitor indicated for the treatment of metastatic castration-resistant prostate cancer (mCRPC). It is jointly developed and manufactured by Medivation and Astellas Pharma.
In December 2010, the results of the Phase III study, COU-AA-301, indicated that abiraterone was effective in improving the overall survival rate among patients. The results indicated that patients who were administered abiraterone demonstrated significant improvements in secondary end points compared to the placebo group and those who were administered prednisone or prednisolone. The trial was sponsored by Ortho Biotech Oncology Research & Development.
Abiraterone is currently undergoing a second Phase III trial, COU-AA-302, initiated in April 2009. The trial is assessing the safety and efficacy of abiraterone in combination with prednisolone in prostate cancer patients who have undergone hormone therapy but not chemotherapy. The study is being carried out on asymptomatic or mildly symptomatic patients with metastatic castration-resistant prostate cancer. The FDA approval of Zytiga to treat mCRPC patients before the use of chemotherapy is based on the results of the second Phase III clinical trial.
The trial includes 1,000 patients across 150 study centres in North America, Europe and Australia. The entire study is expected to be completed by April 2014.
Marketing Zytiga (abiraterone acetate) in the US and worldwide
Improving treatment options, especially for men whose cancer advances despite initial treatment, is an important objective of current drug-related research into prostate cancer. New treatments are especially needed for patients who fail first-line therapy with currently available hormone therapies and develop so-called HRPC or castration-resistant prostate cancer. Data from the abiraterone trials, albeit on relatively small numbers of patients to date, suggest that secondary hormonal therapy may be beneficial in patients failing initial hormone therapy.
The drug has the potential for several other treatment settings, including:
- As first-line therapy in treatment-naive patients suitable for hormone therapy
- As second-line therapy in HRPC
- As third-line therapy in HRPC patients also failing docetaxel-based chemotherapy.
Zytiga (abiraterone) is marketed in the US by Centocor Ortho Biotech. Janssen Pharmaceutical Companies is marketing it in the rest of the world.