ELAHERE® (mirvetuximab soravtansine) is a novel antibody-drug conjugate (ADC) indicated for the treatment of adult patients with folate receptor alpha (FRα)-positive, platinum-resistant epithelial ovarian cancer and other related malignancies, who have undergone one to three previous systemic therapy courses.
ELAHERE was developed by ImmunoGen, a biotechnology company based in the US.
AbbVie, a US-based pharmaceutical company, acquired ImmunoGen in February 2024, which added ELAHERE to its portfolio.
The drug is AbbVie’s first approved solid tumour therapy after the acquisition.
ImmunoGen collaborated with Huadong Medicine, a pharmaceutical company based in China, to develop and market the drug across mainland China, Hong Kong, Macau, and Taiwan in October 2020.
In August 2023, ImmunoGen also collaborated with Takeda Pharmaceuticals, a pharmaceutical company based in Japan, to develop and commercialise the drug in Japan.
Roche’s companion diagnostic, VENTANA FOLR1 RxDx Assay received the US Food and Drug Administration (FDA) approval in November 2022 to detect elevated FRα levels in patients with epithelial ovarian cancer for establishing their suitability for ELAHERE therapy.
ELAHERE is supplied as a sterile injectable solution, that is clear to slightly opalescent and colourless, in a single-dose vial for intravenous administration.
Each vial contains 100mg/20ml of mirvetuximab soravtansine.
Regulatory approvals for ELAHERE
The FDA granted accelerated approval to ELAHERE in November 2022, which was converted into full approval in March 2024.
The European Medicines Agency accepted the marketing authorisation application for ELAHERE to review in October 2023.
Regulatory submissions for ELAHERE are currently being reviewed in several other countries.
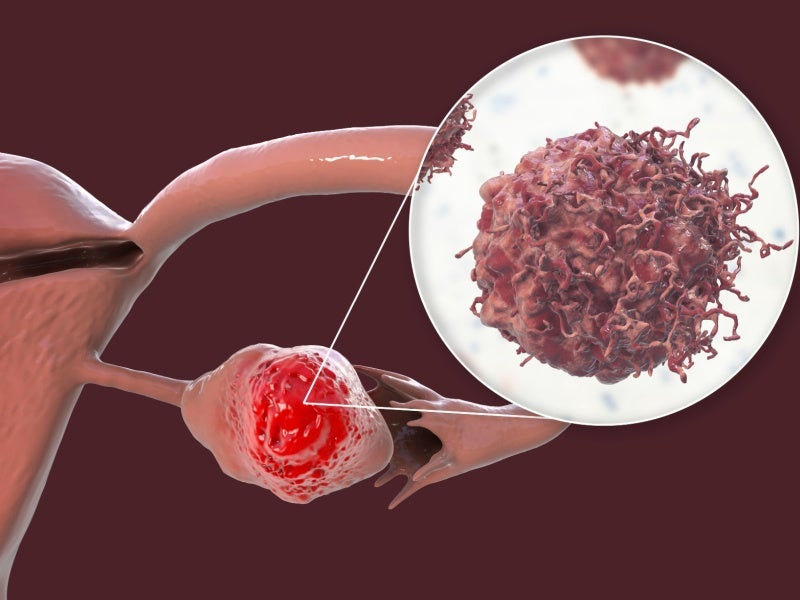
Ovarian cancer causes and symptoms
Ovarian cancer is the most common type of gynaecological cancer, predominantly affecting women in their postmenopausal stages.
The cancer arises due to the rapid growth of tumours within the cells of the female reproductive system.
Ovarian cancer is challenging to detect in its initial stages, thereby complicating treatment efforts. Around 20,000 patients are diagnosed with the disease every year.
The common causes are gene mutations, endometriosis, and previous radiotherapy treatments. Symptoms include a swollen abdomen, discomfort in the pelvic area, and frequent urination.
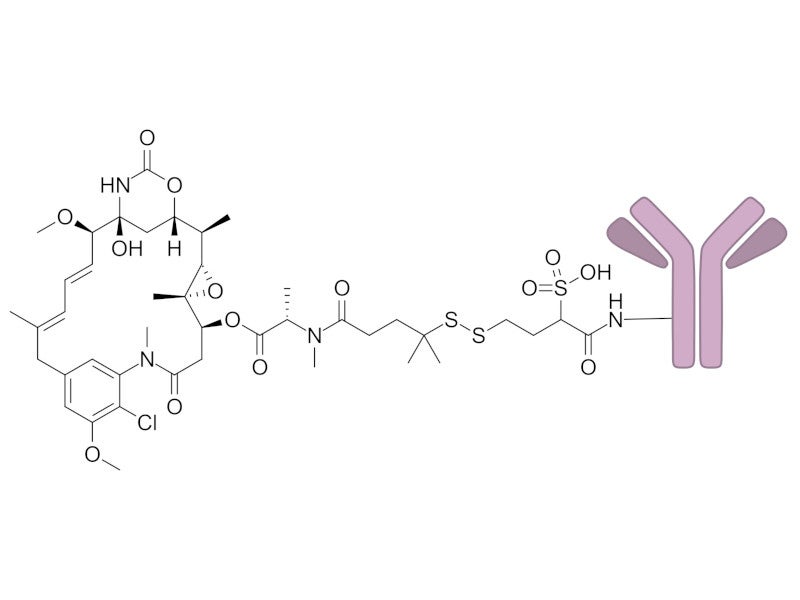
ELAHERE’s mechanism of action
ELAHERE comprises an antibody, which is a chimeric IgG1 directed against FRα.
Maytansinoid DM4, the drug payload in the ADC, is a small molecule microtubule inhibitor attached to the antibody via a cleavable linker.
FRα is a protein present on the surface of certain cancer cells, they serve as a biomarker to determine the appropriateness of targeted treatments.
Upon FRα binding, mirvetuximab soravtansine is internalised, leading to the intracellular release of DM4 through proteolytic cleavage.
DM4 then disrupts the microtubule network within the cell, causing cell death.
Clinical trials on ELAHERE
The FDA’s full approval of the drug is based on the results from the confirmatory Phase III MIRASOL trial.
The randomised clinical trial was a comparative study between ELAHERE and the investigator’s choice (IC) of single-agent chemotherapy, which included weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan.
The eligibility criteria for the trial were patients with platinum-resistant ovarian cancer exhibiting high levels of FRα expression.
The primary endpoint of the trial was progression-free survival, as assessed by the investigator.
A total of 453 patients were enrolled and graded according to the number of prior lines of therapy and the IC chemotherapy received.
The results indicated a 35% reduction in the risk of cancer progression in patients treated with ELAHERE compared to IC chemotherapy.
The median PFS for patients treated with ELAHERE and IC chemotherapy was 5.62 months and 3.98 months, respectively.
The overall response rate in the ELAHERE arm was 42.3%, with 12 complete responses, compared to 15.9% in the IC chemotherapy arm, which had no complete responses.
A few common adverse events reported in patients include blurred vision, nausea, elevated liver enzymes, peripheral neuropathy, reduced platelet counts, decreased magnesium levels, and lowered leukocyte counts, among others.
Additional clinical trials on ELAHERE
AbbVie is also conducting trials to explore the efficacy of ELAHERE in platinum-sensitive ovarian cancer.
The drug is being evaluated in the Phase III GLORIOSA trial in combination with bevacizumab (Avastin) and as a monotherapy in the Phase II PICCOLO trial, where it achieved the primary endpoint with an overall response rate of 51.9%.