Under development by Eli Lilly, LY2140023 is a novel investigational agent for the treatment of schizophrenia. A member of a new class of antipsychotic drugs, LY2140023 is still in early stage clinical development. Nevertheless, analysts have hailed it as an exciting new medicine that may herald the arrival of third-generation antipsychotic drugs.
In March 2009, Lilly announced inconclusive results from its HBBI Phase II clinical trial. A higher-than-expected placebo response approximately double of that normally witnessed in schizophrenia clinical trials was observed in the trial.
Despite inconclusive clinical trial results, Lilly will continue developing the drug as such results are common in the area of neuroscience.
Since an earlier Phase II trial showed a positive response in 2007, Lilly is conducting an additional Phase II study, HBBM, to test the safety and efficacy of LY2140023.
Lilly initiated Phase III clinical trials on LY2140023 in March 2011.
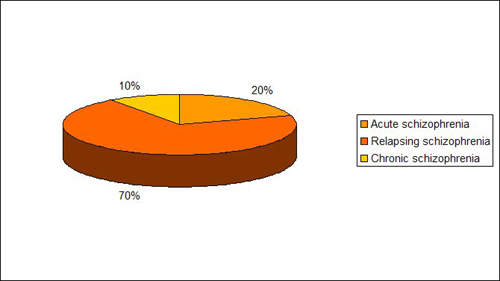
Bridging treatment gaps in schizophrenia
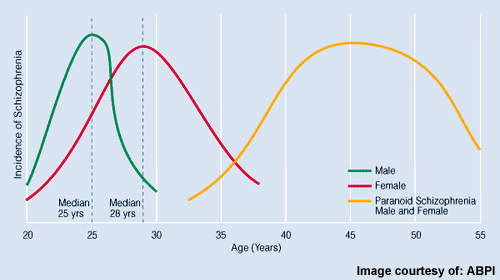
Estimated to affect about 1% of the general population, schizophrenia is a serious and devastating mental illness. Antipsychotic drugs are the mainstay of treatment and designed to ameliorate the core symptoms of the illness, which include:
- Positive symptoms – hallucinations, delusions, agitation, disorganised thinking
- Negative symptoms – introversion, apathy, low self-esteem leading to personal neglect and more rarely catatonia
- Cognitive symptoms – poor memory, attention deficit, executive dysfunction
- Affective symptoms – depression , elation, suicidal ideation
Although an expanding range of antipsychotic drugs has greatly improved treatment of schizophrenia, there remain significant treatment gaps. New antipsychotic drugs are needed to provide broader efficacy to treat patients refractory to current agents; safer drugs are needed to improve treatment compliance.
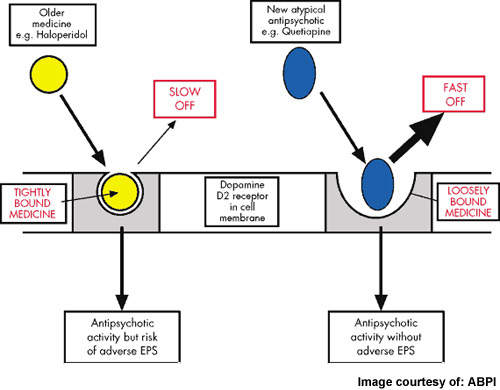
Atypical antipsychotics, which account for over 90% of the market today, offer improved safety and tolerability over the older conventional typical antipsychotics. However, significant weight gain leading to metabolic disturbances (diabetes, heart disease), sexual dysfunction, hyperprolactinaemia and cardiotoxicity are common side-effects associated with many atypical drugs. Poor tolerability leads to poor long-term adherence to treatment and increased risk of relapse.
Targeting glutamate in antipsychotic drug research
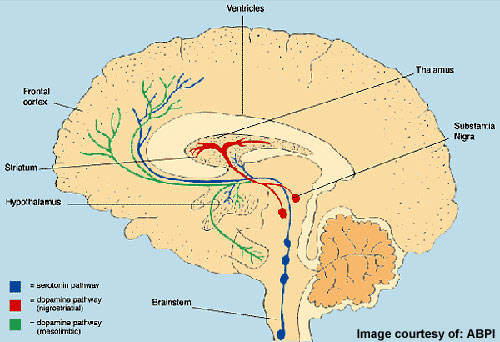
All currently available antipsychotic drugs block dopamine, D2, receptors in the brain, with D2 receptor antagonism considered the primary mechanism by which they alleviate positive symptoms of schizophrenia. Some also act on serotonin receptors, which may contribute to efficacy against negative symptoms.
Lilly’s LY2140023 differs from established antipsychotic drugs in that it targets glutamate rather than dopamine or serotonin receptors. There is a substantial body of evidence to suggest that glutamate, a neurotransmitter, may also be involved in the pathophysiology of schizophrenia.
For years, psychiatrists have known that the psychomimetic street drug phencyclidine (PCP) can induce symptoms almost identical to those of schizophrenia. PCP acts as a NMDA glutamate receptor antagonist to non-competitively block the NMDA subtype of the glutamate receptor and thus impair brain-signalling pathways dependent on glutamate.
Research into glutamate drugs for schizophrenia has focused on compounds that can enhance NMDA receptor function as well as those that can reduce glutamate release.
LY2140023, an oral prodrug, is metabolised to the active mGlu2/3 receptor agonist LY404039, which is believed to work by reducing the presynaptic release of glutamate in those regions of the brain where mGlu2/3 receptors are expressed.
LY2140023 appears efficacious and well tolerated
Encouraging evidence of the antipsychotic effects of LY2140023 has been demonstrated in a Phase II proof of concept study. In this four-week, double-blind study, 196 patients with schizophrenia were randomised to treatment with LY2140023 (40mg twice daily), olanzapine (15mg daily) or placebo.
In total, 118 patients completed the four-week study at the end of which those in the LY2140023 and olanzapine groups demonstrated significantly greater response rates compared with placebo recipients (32.0% and 41.2% vs. 3.2% respectively; p<0.001). A response was defined as ≥25% decrease in Positive and Negative Syndrome Scale (PANSS) total score.
Overall, LY2140023 appeared safe and well tolerated. Most adverse events were mild-to-moderate in severity and not treatment-limiting. Importantly, LY2140023 appeared devoid of certain adverse events that are common with existing antipsychotic drugs, including hyperprolactinaemia, extrapyramidal symptoms and weight gain.
Following positive results from Phase II, Lilly launched larger-scale Phase II trials to test the safety and efficacy of the drug. Lilly recruited 669 patients for the HBBI clinical trial, however, only 393 of them completed it. The four-week in-patient trial did not show any of the four administered LY2140023 monohydrate doses separated from the placebo. The dose was well tolerated and did not show adverse weight gain.
Eli Lilly is planning to launch a multi-centre, double-blind and placebo-controlled Phase III study on LY2140023 in March 2011. The trial will enrol 950 patients and is expected to be concluded by February 2013.
The study will determine efficacy of at least one dose level of LY2140023 compared to placebo. LY2140023 will be administered twice daily in 10mg, 40mg and 80mg doses for seven weeks. The primary outcome measure of the study will be to determine the change in total score of PANSS from the baseline.
Marketing commentary
All currently available atypical antipsychotic agents target dopamine receptors in the brain. The fact that not all patients respond to existing antipsychotic drugs suggests that other neurotransmitters may be involved in the pathogenesis of schizophrenia.
Glutamate has been the focus of research into schizophrenia for many years and this research may now be bearing fruit with the development of drugs, such as LY2140023, that target glutamate receptors.
While preliminary clinical data on this experimental glutamate agonist suggest that modulating glutamate receptors may control symptoms of schizophrenia, much more clinical data are needed to substantiate these early findings. A larger, 870-patient study is already scheduled to determine the optimum dose of LY2140023.