ELREXFIO™ (elranatamab) is an off-the-shelf (ready-to-use) fixed-dose bispecific monoclonal antibody indicated for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who have undergone at least four previous therapy lines.
Developed by the pharmaceutical company Pfizer, the drug was launched in Japan under the brand name Elrefio® in May 2024.
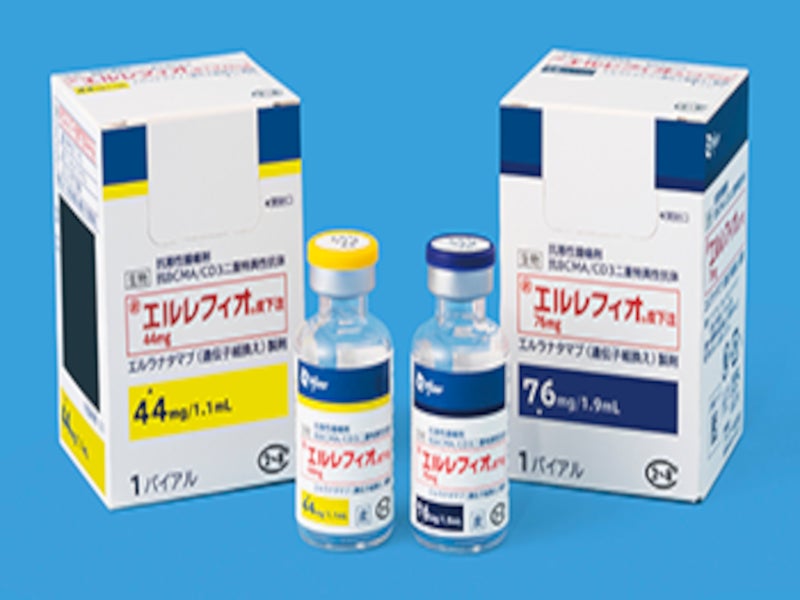
ELREXFIO is available as a clear to slightly opalescent, and colourless-to-pale-brown liquid solution in dosage strengths of 76mg/1.9ml and 44mg/1.1ml in single-dose vials for subcutaneous injection-
Regulatory approvals for ELREXFIO
The US Food and Drug Administration (FDA) granted ELREXFIO accelerated approval for the treatment of RRMM in August 2023. Following this, the drug received conditional marketing authorisation in Europe in December 2023 and was subsequently approved in Japan in March 2024.
ELREXFIO is also approved in Switzerland, Brazil, and Canada under Project Orbis, a framework that allows for the simultaneous submission and review of cancer drugs among international partners to accelerate approvals
Additionally, the Medicines and Healthcare products Regulatory Agency (MHRA) has authorised ELREXFIO for use in Great Britain for RRMM.
Multiple myeloma causes and symptoms
Multiple myeloma is a severe and incurable blood cancer that compromises the body’s ability to fight infection by affecting plasma cells in the bone marrow. It is the second most prevalent blood cancer in Europe, with more than 50,000 new cases diagnosed annually, contributing to a global total exceeding 187,000 new diagnoses each year.
Approximately 40% of patients with multiple myeloma do not survive beyond five years, and the majority will experience relapse, necessitating multiple lines of therapy.
The primary therapeutic objective for RRMM patients is to manage the disease effectively while minimising toxicity and enhancing quality of life.
ELREXFIO’s mechanism of action
ELREXFIO is a bispecific B-cell maturation antigen (BCMA)-directed monoclonal antibody. It operates by simultaneously binding to BCMA on plasma cells, plasmablasts and multiple myeloma cells, as well as to CD3 on T-cells. This dual binding action triggers the cytolysis of BCMA-expressing cells.
The drug’s engagement with T-cells leads to the release of proinflammatory cytokines, culminating in the lysis or disintegration of multiple myeloma cells.
Clinical studies on ELREXFIO
The MagnetisMM-3 trial, a phase II, open-label, single-arm, multi-centre study, has been pivotal in the approval of ELREXFIO for the treatment of RRMM. The study assessed the drug’s efficacy and safety in a cohort of patients resistant to key myeloma therapies, including at least one immunomodulatory agent, one proteasome inhibitor and one anti-CD38 monoclonal antibody.
Participants were stratified into two groups, cohort A, comprising patients naive to BCMA-targeted therapy, and cohort B, consisting of individuals previously treated with BCMA-targeted therapy or chimeric antigen receptor (CAR) T-cell therapy. The primary endpoints were response rates and the duration of response.
ELREXFIO was administered subcutaneously, starting with a two-step priming dose of 12mg on day one and 32mg on day four, followed by 76mg weekly for 28 days. The drug demonstrated significant efficacy in cohort A, with an overall response rate of 58% among patients who had undergone four or more prior lines of therapy. 82% of these responders maintained their response for at least nine months, with the median time to first response being 1.2 months.
ELREXFIO has been distinguished as the first BCMA-directed therapy in the US to offer bi-weekly dosing for patients who respond after 24 weeks of weekly treatment. This advancement promises reduced clinic visits and potentially enhanced long-term tolerability.
Cohort B’s data also features in the label, revealing an overall response rate of 33% in patients who had received at least four prior lines of therapy, including a BCMA-directed therapy. After a median follow-up of 10.2 months, an estimated 84% of these patients sustained their response for no less than nine months.
The most frequently observed adverse events in the clinical trial included cytokine release syndrome, anaemia, neutropenia, diarrhoea, thrombocytopenia, fatigue, decreased appetite, lymphopenia, injection site reactions, pyrexia, nausea and hypokalaemia.
Pfizer is progressing the MagnetisMM clinical programme to extend the use of ELREXFIO™ into earlier lines of treatment, both as a monotherapy and in combination with standard or novel therapies.