Erwinaze (asparaginase Erwinia chrysanthemi) is indicated for the treatment of acute lymphoblastic leukaemia (ALL). It is manufactured by EUSA Pharma.
It was developed jointly in collaboration with the UK’s Health Protection Agency (HPA) and AIBioTech.
In November 2010 EUSA Pharma submitted a Biologics License Application (BLA) for erwinaze to the US Food and Drug Administration (FDA). The FDA accepted the application and granted a priority review status to the drug in January 2011.
The FDA approved the drug in November 2011 for use in ALL patients who have developed hypersensitivity to E. coli-derived asparaginase. The drug also received approval in Canada, the UK and several European Union member countries.
Details of acute lymphoblastic leukaemia (ALL) and new case rates
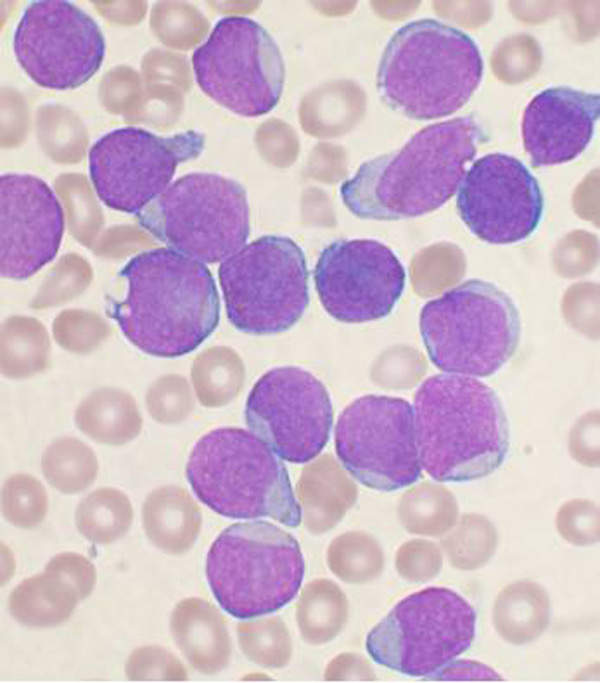
Acute lymphoblastic leukaemia is a type of cancer which affects the white blood cells in the body. It is characterised by the production of excess lymphocytes in the bone marrow.
It prevents the formation of healthy white blood cells and replaces normal red blood cells with cancerous cells. The fatal disease starts at the bone marrow and spreads to other organs in the body.
Every year more than 4,000 new cases of ALL get registered in the US and about 400 in the UK. The disease commonly affects children younger than ten years of age.
How erwinaze’s mechanism of action prevents growth of leukaemia cells
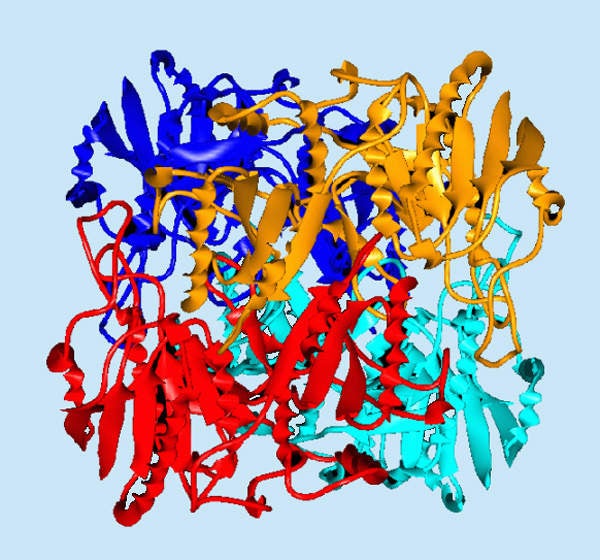
The drug contains an asparaginase enzyme derived from Erwinia chrysanthemi. The enzyme in the drug reduces the level of asparagine in the bloodstream. The drug prevents the growth of leukaemia cells by limiting asparagines in the blood.
It also stops the growth of cells associated with acute lymphoblastic leukaemia. The drug is supplied in injection form through intramuscular administration.
Clinical trials on EUSA Pharma’s ALL-treatment drug
Phase I clinical trials on erwinaze were conducted between June 2006 and December 2011. It was a non-randomised, open label and single group study intended to discover the safety and efficacy of the drug. The study enrolled 87 patients.
An interventional clinical trial on erwinaze was conducted between February 2008 and February 2011. It was conducted on 55 patients to find out the pharmacology and toxicity of the drug.
The primary outcome measure was determining whether serum asparaginase activity is ≥ 0.1 IU/mL in ALL patients. The secondary outcome measures included comparison of erwinaze with PEG-asparaginase treated patients and determining serum asparaginase activity after 72 hours.
The FDA-approval of erwinaze was based on phase III clinical trial. It was a single-arm, open label, multicentre clinical trial. It enrolled 58 patients with ALL who were not able to continue using pegaspargase due to the hypersensitivity reactions.
The patients were administered with erwinaze 25,000 International Units/m2 for two weeks instead of each scheduled pegaspargase dose. The primary endpoint of the study was determining the patients who achieved a serum trough an asparaginase level greater than or equal to 0.1 International Units/mL. The study results showed that more than 50% of the patients reached the endpoint after administering the third dose of the drug.
The FDA also collected additional safety data on erwinaze from clinical trials on more than 843 patients. The common side effects observed in the clinical trials included severe allergic reactions, high liver enzymes, pancreatic inflammation, abnormal bleeding or clotting, high blood sugar and nausea.
Marketing commentary for erwinaze and similar FDA-approved drugs
In July 2010, EUSA Pharma granted the commercial rights on erwinaze in Japan to Ohara Pharmaceutical. Erwinaze was classed as an orphan drug by the FDA in the US. It is now commercially available in the US.
The FDA approved two other drugs for the same indication, which include Elspar (asparaginase injection) and Oncaspar (pegaspargase). Both the drugs were derived from E. coli.