Developed by US biopharmaceutical company Esperion Therapeutics, ETC-216 is a synthetic variant of high-density lipoprotein (HDL) and one of several HDL targeted therapies the company is developing for patients with CHD. Unlike established treatments for dyslipidaemia, Esperion Therapeutics’ drugs are designed to enhance a process known as ‘reverse lipid transport’ in which HDL is used to clear fatty plaque from arteries.
This novel phospholipid therapy has attracted considerable interest following publication of promising results in a phase II clinical trial in which it showed evidence of inducing regression in fatty plaques or atheromas. These results, albeit based on a small sample of patients, represented the first time drug treatment had shown evidence of not only preventing progression of atherosclerosis in patients with CHD but also reducing the build-up of plaque.
Following Pfizer’s acquisition of Esperion, along with the rights over ETC-216 in 2004, the trials on the drug were not advanced due to Pfizer’s priority to develop its oral drug torcetrapib. The company also suspended Esperion’s research activities on the ETC-216 in Ann Arbor in 2007.
However, Pfizer sold Esperion back to an investor syndicate in May 2008 for $22.75m. The syndicate included Aisling Capital, Alta Partners and Domain Associates and Arboretum Ventures. Roger Newton, the co-founder of Esperion was made CEO and president of the new company and this is expected to pave way for resuming the clinical studies on ETC-216.
HDL targeted therapies represent a new treatment approach for CHD
The introduction of the statins, drugs that reduce plasma levels of atherogenic lipids and lipoproteins in particular low-density lipoprotein cholesterol (LDL-C), has revolutionised the treatment of dyslipidaemia. Now attention is focusing on therapies that can boost the levels of cardioprotective lipoproteins, such as HDL.
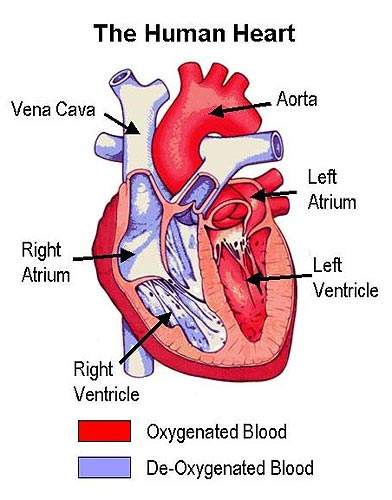
In contrast to LDL, which transports cholesterol from the liver to other parts of body where it is used to make hormones and cell membranes, HDL transports excess cholesterol and other lipids to the liver for elimination from the body.
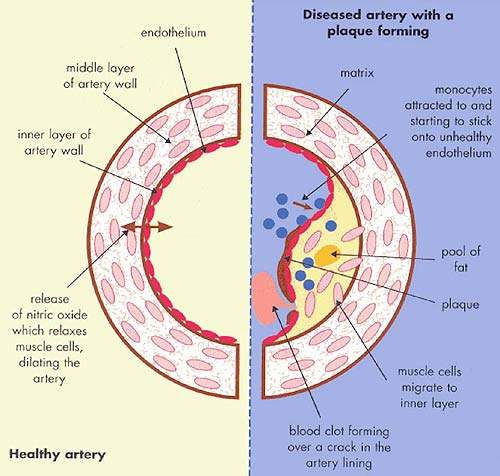
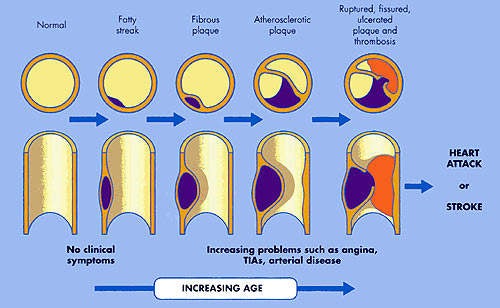
It is the build up of fatty material in the artery wall that leads to atherosclerosis, a condition characterised by progressive narrowing of the arteries supplying blood to the heart.
By removing excess cholesterol from the circulation, HDL can prevent arteries from becoming clogged with fatty material.
Although statins increase levels of cardioprotective HDL, the effect is relatively modest in comparison with their capacity to reduce LDL-C, hence the interest in alternative therapies for raising HDL levels.
Clinical trials suggest ETC-216 may reverse atehrosclerosis
The phase II clinical trial that has attracted media interest was carried out in 57 patients with acute coronary syndrome (ACS), who were randomised to five-weekly infusions of either ETC-216, at one of two doses, or placebo (saline). Intravascular ultrasound (IVUS) was performed within two weeks of patients experiencing ACS (baseline) and after each weekly infusion. The primary outcome measure was the change from baseline in percentage atheroma volume, as measured by IVUS, in the combined ETC-216 cohort. Results showed that five weeks of treatment with ETC-216 produced a significant, 4.2% reduction in atheroma volume (p<0.01).
Trial investigators in the US have described the results of the phase II study as ‘unprecedented’ and the best example to date that directly targeting HDL offers therapeutic benefit in patients with CHD.
Acquisition of Esperion to strengthen Pfizer’s cardiovascular portfolio
Pfizer, already a dominant force in the CHD market with its statin Lipitor (atorvastatin) for the treatment of dyslipidaemia, announced towards the end of 2003 that it was to acquire Esperion Therapeutics. This development was aimed at strengthening Pfizer’s range of cholesterol products, which also included its cholesterol ester transfer protein (CETP) inhibitor, torcetrapib (CP-529,414), a drug which raises HDL by a different mechanism to ETC-216, and the ACAT inhibitor avasimbe.
Interestingly, Pfizer already had an option to co-market ETC-216 following its recent merger with Pharmacia, with which Esperion had formed an alliance for the product in 1998.
Acquisition of Esperion was expected to bring not only ETC-216 into the Pfizer fold but also ETC-588, also in Phase II development, ETC-642 and several other early-stage HDL products.
Esperion’s ETC-216 differs from Pfizer’s current cholesterol drugs in that it is designed to be used intravenously following myocardial infarction or other major coronary events.
It was envisaged that ETC-216 would be used initially as a short-term treatment following ACS to lower plaque burden, after which patients would be switched to long-term maintenance therapy with oral agents, such as Pfizer’s atorvastatin-torcetrapib combination therapy, then in advanced clinical development. If its early promise was sustained in larger-scale Phase III trials and the product eventually approved, ECT-216 could have complemented rather than compete with torcetrapib.
Marketing commentary
Coronary heart disease (CHD), the physical manifestation of atherosclerosis, is a major cause of death and disability. Of the 17 million deaths that occur from cardiovascular disease in the world each year, CHD is a significant contributor. Statins, used to treat dyslipidaemia, dominate the market for cardiovascular drugs, with Pfizer’s Lipitor (atorvastatin) currently the world’s top-selling medicine.
Potentially, drugs which target HDL may become as important as the statins, which large-scale intervention trials have shown significantly reduce CHD-related morbidity and mortality. While proof-of-concept has now been established for ETC-216 it remains to be seen if the short-term changes in plaque volume, as measured by IVUS, translate into longer-term clinical benefits. Questions that need to be addressed are whether drugs such as ETC-216 can reduce the risk of coronary events and the need for re-hospitalisation as well as decrease the need for coronary angioplasty.