Faslodex (fulvestrant) is an oestrogen receptor antagonist indicated for the treatment of locally advanced or metastatic breast cancer.
Discovered and developed by AstraZeneca, Faslodex was first approved by the US Food and Drug Administration (FDA) in 2002 as a monotherapy for the treatment of hormone receptor-positive (HR+) metastatic breast cancer.
The drug is also approved as a combination therapy with CDK4/6 inhibitor palbociclib for the treatment of HR+ and human epidermal growth factor receptor 2 negative (HER2-) advanced or metastatic breast cancer patients previously treated with endocrine medicine.
Faslodex was approved as first-line therapy by the European Commission (EC) in July 2017 and as a combination therapy with palbociclib in November 2017 in breast cancer patients that received prior endocrine therapy.
A new indication for the drug in combination with CDK4/6 inhibitor abemaciclib was approved by the FDA in November 2017 for the treatment of HR+ and HER2- in patients previously treated with endocrine therapy.
Faslodex is also approved in 80 countries worldwide and is being tested as a combination drug with 19 different medicines for the treatment of women with advanced HR+ breast cancer.
Advanced or metastatic breast cancer causes
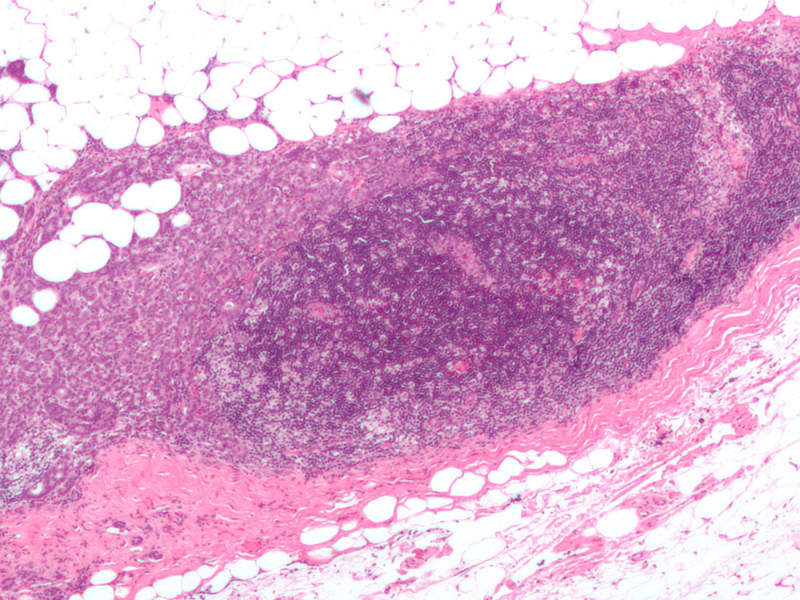
Also known as locally-advanced breast cancer, advanced or metastatic breast cancer occurs when cancer cells spread beyond the original tumour site to other parts of the body outside of the breast.
Symptoms include bone pain, skin ulcers, swelling of the lymph node in the armpit, breast pain, and unusual weight loss.
An estimated 153,000 women in the US are living with advanced-stage breast cancer, which is expected to increase to 160,000 by 2020.
While the disease currently has no cure, existing treatments delay the progression to maintain the quality of life of patients.
Faslodex’s mechanism of action
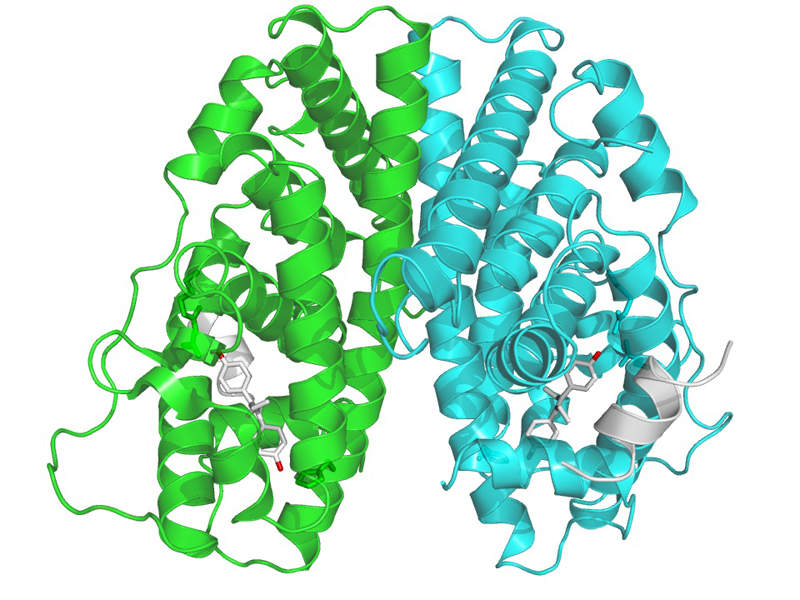
Faslodex contains an oestrogen receptor antagonist, which binds to the oestrogen receptor and downregulates the endoplasmic reticulum (ER) protein in human breast cancer cells.
The drug is available in 500mg injection for intramuscular use.
Clinical trials on Faslodex
The FDA’s approval for Faslodex in combination with abemaciclib was based on data obtained from a Phase III clinical trial named MONARCH 2. It was a randomised, double-blind, placebo-controlled, multi-centre study that enrolled 669 women with HR+ and HER2- advanced breast cancer.
The primary endpoint of the study was progression-free survival (PFS). Its results demonstrated that the patients who were administered with Faslodex 500mg and abemaciclib 150mg witnessed 16.4 months median PFS compared to 9.3 months in Faslodex and placebo group.
The first-line therapy approval of Faslodex by the EC was based on results from a Phase III clinical study named Fulvestrant and AnastrozoLe compared with hormonal therapy naive advanced breast cancer (FALCON).
This study was a randomised, double-blind, multi-centre trial that compared Faslodex 500mg plus placebo with anastrozole 1mg plus placebo in post-menopausal women with HR+, locally advanced or metastatic breast cancer who were not treated with any hormonal medicine.
The study results showed that the patients administered with Faslodex 500mg had median PFS of 16.6 months compared with 13.8 months for anastrozole plus placebo.
The EC approval for Faslodex in combination with palbociclib was based on results obtained from a Phase III clinical trial named PALOMA-3. It was a randomised, double-blind, parallel group, multi-centre study conducted on 521 women patients with HR+/HER2- advanced or metastatic breast cancer across 140 sites worldwide.
The patients were administered with Faslodex plus palbociclib versus fulvestrant plus placebo in a 2:1 ratio. The study’s results showed that patients treated with Faslodex 500mg and palbociclib 125mg had median PFS of 9.5 months compared to 4.6 months in those treated the Faslodex and placebo.