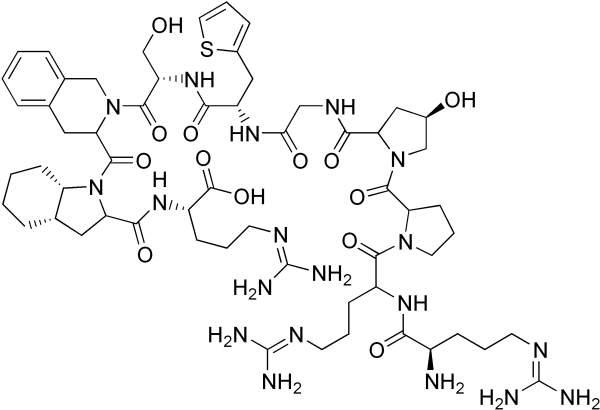
FIRAZYR (Icatibant) is a drug developed and manufactured by Jerini, which was taken over by Shire Pharmaceuticals in 2008. The drug is indicated for the treatment of Hereditary Angioedema (HAE). It is available in 3ml pre-filled syringes.
The drug was granted orphan drug designation in the EU in February 2003. It also received orphan drug designation in US and Australia.
A marketing authorisation application was submitted to the European Medicines Agency (EMA) in July 2007. EMA approved the drug for the treatment of hereditary angioedema in adults in July 2008.
Firazyr was approved by the Australian Therapeutic Goods Administration in June 2010.
In October 2007, Jerini submitted a new drug application of Firazyr to the US FDA. The drug was rejected by the FDA in April 2008.
Shire submitted a complete response letter to the FDA in February 2011 and the drug was consequently approved for acute attacks of HAE in August 2011.
Firazyr is currently approved in 37 countries including the US, EU, Australia, Argentina, Brazil, Israel and Denmark.
Hereditary Angioedema
HAE is a life-threatening genetic disorder. Patients suffer from recurrent swelling in various parts of the body. Swelling can be observed in hand, feet, face, larynx and abdomen.
HAE on the hands, feet and face may completely disfigure those parts of the body. HAE of the gastrointestinal tract leads to severe pain due to the swelling in intestinal wall.
Swelling in the larynx blocks the upper airways, which may lead to suffocated death. It is estimated that one out of every 50,000 people is inflicted with HAE in the EU and one in every 10,000 suffers from HAE in the US. Approximately 15,000 and 75,000 people are affected by HAE in the EU and the US respectively.
Firazyr – drug mechanism of action
Hereditary angioedema occurs due to absence of an inherited protein of Factor XII called the C1-esterase-inhibitor. The absence of C1-esterase causes bradykinin production.
Bradykinin causes blood vessels to widen further, leading to inflammation, swelling and pain. Bradykinin is considered to be the cause of the symptoms (swelling, inflammation and pain) occurring in HAE patients.
Firazyr is a bradykinin B2 receptor antagonist. It hinders bradykinin from binding to B2 receptors and thereby stops the progression of swelling (Oedema) and reduces the symptoms of HAE attack.
Clinical trials of Firazyr / Icatibant
A Phase II/III study named FAST- 1 was initiated in August 2004 in 56 HAE patients. The trial was conducted to evaluate the safety and efficacy of Firazyr in the treatment of HAE. The trial data was released in March 2008.
The Phase III trial FAST-2 was initiated in March 2005. The trial was conducted to evaluate the safety, efficacy and tolerability of the drug in HAE patients. It enrolled around 80 HAE patients and was completed by March 2008.
In both the trials, FAST 1 and FAST 2, the drug was observed to be tolerable and efficient in the treatment of laryngeal attacks.
Around 60 patients with laryngeal attacks were treated. The efficacy results were similar in patients with laryngeal attacks as well as those with non-laryngeal attacks. Around a 50% reduction in symptoms was achieved in a median time of two to 2.3 hours.
In June 2009 Shire initiated FAST-3, a Phase III trial, to evaluate the efficacy of the drug and compare it to that of a placebo. The trial enrolled 98 HAE patients at 67 sites in nine countries. The patients were administered either 30mg of Icatibant or given a placebo.
The top-line results were released in December 2010. An onset time of symptom relief was observed at 1.5 hours compared to 18.5 hours in the case of placebo.
The median time for the reduction of symptoms in cutaneous or abdominal attacks was two hours when compared to 19.8 hours in the case of a placebo. The entire study is expected to be completed by June 2012.
Around 150 patients were recruited for a Phase IIIb trial for the evaluation of the safety and efficacy of self administration in HAE patients. The patients were administered a single dose of 30mg Icatibant.
The trial began in September 2009 and was completed by June 2011. The self-administration was observed to be well tolerable. Time to symptom relief was same in both self administered patients and those administered by a professional.
Another Phase III trial was initiated in September 2011 to evaluate the pharmacokinetics, tolerability and safety of the drug in paediatric HAE patients. A total of 36 children aged between two and 17 years-old have been enrolled. The patients will be administered 04mg/kg of the drug up to a maximum dose of 30mg subcutaneously. The trial is scheduled to be completed by September 2014.