Galafold™ (migalastat) is indicated for the treatment of adult patients aged 16 years and older with Fabry disease.
Developed by US-based biopharma company Amicus Therapeutics, Galafold™ is one of the first oral medications for Fabry disease.
Amicus submitted a new drug application (NDA) for migalastat to the US Food and Drug Administration (FDA) in December 2017, which was accepted for priority review in February 2018. The drug was approved in August 2018.
Galafold™ is already approved in Australia, Canada, the EU, Israel, Japan, South Korea and Switzerland, and is awaiting approval in Taiwan.
The drug approved by the Agency of Medicines, Food and Medical Devices (ANMAT) in Argentina in August 2019.
Fabry disease causes and symptoms
Fabry disease is a rare and progressive genetic disorder that occurs due to mutations in the Galactosidase Alpha (GLA) gene. The defective gene causes a deficiency of the alpha-galactosidase A (a-Gal A) enzyme, which is particularly active in lysosomes.
The disease causes lysosomes to malfunction and leads to the accumulation of a fatty substance called globotriaosylceramide (GL-3) in the body. The accumulated fat affects various parts of the body, including the eyes, kidneys, heart, brain and nervous and gastrointestinal systems.
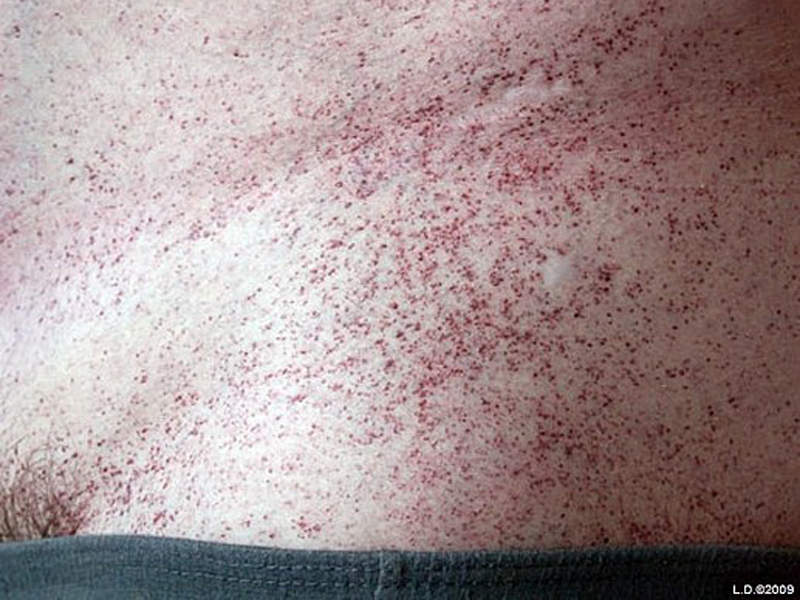
Common symptoms of the disease include a pain and burning sensation in the hands and feet, angiokeratoma (red spots on the skin), cloudy vision, ringing in the ears, hearing loss, joint pain and kidney problems. The symptoms affecting the kidney, heart or brain mostly show in patients aged 30 to 45 due to the progressive nature of the disease.
Fabry disease occurs mostly in males and rarely in females. It affects approximately one in 40,000 males in the US, adding up to around 3,000 people.
Galafold (migalastat) mechanism of action
Galafold™ reversibly binds to the active site of deficient alpha-Gal A proteins in the body and stabilises dysfunctional enzymes. This helps the lysosomes in clearing the accumulated disease, causing fatty substance from the body.
The drug is available as an oral alpha-Gal A pharmacological chaperone in capsule form in 123mg migalastat strength.
Clinical trials on Galafold
The FDA’s approval of Galafold™ was based on the positive results of a phase three pivotal clinical trial named Study 011 or FACETS. A total of 67 previously untreated patients with Fabry disease participated in the trial, which was carried out in three phases. Out of 67 patients, 50 had amenable GLA variants.
The first phase was a six-month randomised, double-blind, placebo-controlled study that saw patients randomised to either Galafold™ 123mg every alternate day or placebo for six months in a 1:1 ratio. In the next phase, all patients in the clinical trial received Galafold™ for six months.
Results from the study showed that patients administered with Galafold™ showed a 50% or more reduction in the amount of accumulated disease-causing fatty substance in the body, compared to 45% in patients treated with placebo.
The last phase of the study was a 12-month open-label extension phase.
The most common adverse events noticed in patients treated with Galafold™ in the trial included urinary tract infection, headaches, nasopharyngitis, nausea and pyrexia.
Marketing commentary on Amicus Therapeutics
Amicus Therapeutics is a biopharmaceutical company based in Cranbury, New Jersey, US.
The company focuses on the development of advanced therapies for the treatment of a wide range of rare and orphan diseases.
The lead biological programme in the company’s pipeline is AT-GAA, which is under development for the treatment of Pompe disease.