Gardasil 9 (human papillomavirus 9-valent vaccine, recombinant) is indicated for the prevention of cancers caused by some types of human papillomavirus (HPV).
The vaccine was discovered and developed by Merck.
Gardasil 9 was approved by US Food and Drug Administration (FDA) as a preventative vaccine for various types of cancers in December 2014.
The vaccine is indicated for use in girls and young women between the age of nine and 26, and boys between the age of nine and 15 to prevent cervical, vulvar, vaginal and anal cancers caused by HPV types 16, 18, 31, 33, 45, 52 and 58.
It is also indicated for the prevention of precancerous or dysplastic lesions caused by HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58, and genital warts caused by HPV types 6 and 11.
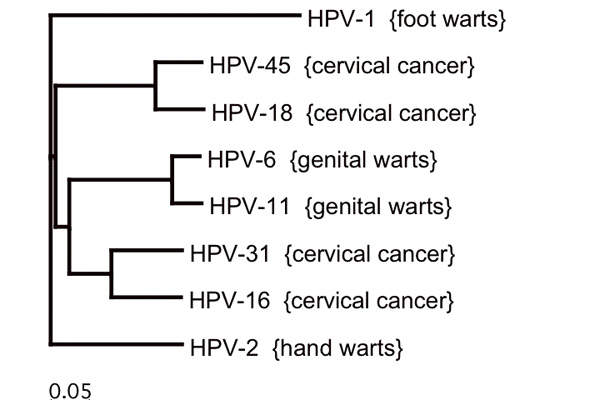
HPV types and disease severity
GSK’s Cervarix is a prophylactic vaccine, indicated for the prevention of precancerous cervical lesions (high-grade cervical intraepithelial neoplasia: CIN grades 2 and 3) and cervical cancer associated with human papillomavirus (HPV) types 16 and 18.
HPV infection causes warts on different parts of the body and may also lead to cancers of the cervix, vulva, vagina, penis, oropharynx and anus. The seven HPV types 16, 18, 31, 33, 45, 52 and 58 cause most of these cancers.
Approximately 575,000 cases of HPV-related cancers are estimated to occur annually worldwide. It is also estimated that 33 new cervical cancer cases are diagnosed each day totalling to 12,000 cases every year in the US.
According to American Cancer Society, approximately 2,600 men and 4,500 women in the US were diagnosed with anal cancer in 2014. An estimated one million cases of genital warts are recorded every year.
US Centers for Disease Control and Prevention (CDC) made HPV vaccination a public health priority. According to the CDC, an additional 53,000 cervical cancer cases could be prevented by increasing three-dose HPV vaccination coverage to 80%.
Gardasil 9 mechanism of action, dosage and administration
The exact mechanism of action of Gardasil could not be established yet as HPV infects only human beings. However, animal studies showed the vaccine could be developing humoral immune responses.
Gardasil 9 is available in the form of a 0.5ml vial for intramuscular use only. The schedule of the vaccine is at the beginning, two months and six months. The drug should be administered intramuscularly in the deltoid region of the upper arm, or in the higher anterolateral area of the thigh.
Clinical trials on Gardasil 9
The FDA approval for Gardasil 9 was based on four clinical trials, which included two Phase II trials and two Phase III trials also known as FUTURE (Females United To Unilaterally Reduce Endo/Ectocervical Disease) I and II clinical trials.
All four clinical studies were placebo-controlled, double-blind, randomised trials, which enrolled a total of 20,541 women between the age of 16 and 26.
Patients were administered with Gardasil 9 vaccine, designed to prevent cervical cancer, cervical dysplasias, vulvar or vaginal dysplasias, or genital warts.
Results of the study demonstrated no new cases of HPV-16 or 18 related cervical intraepithelial neoplasia (CIN) 2/3 or adenocarcinoma in situ (AIS) were reported in patients treated with the full treatment regimen of Gardasil 9, while 53 cases were reported in the placebo group.
Four new cases of HPV-6, 11, 16, or 18 related CIN or AIS were reported in Gardasil 9-administered patients, when compared to 83 for placebo, and one case of HPV-6, 11, 16, or 18 related genital warts in Gardasil 9-group, compared to 91 for placebo.
Adverse events reported by Gardasil 9-administered patients included injection site pain, swelling, and erythema. Fever, nausea, and nasopharyngitis were also reported as adverse events.
Efficacy of Gardasil 9 was assessed in an active comparator-controlled, double-blind, randomised clinical trial (Study 1) conducted on girls and women between the age of 16 and 26.
The study enrolled 14,204 women who were vaccinated without pre-screening for the presence of HPV infection. It compared clinical efficacy of Gardasil 9 and Gardasil for five additional HPV types.
In clinical trials, Gardasil 9 demonstrated 96.7% efficacy against the combined incidence of cervical, vaginal and vulvar cancers caused by HPV types 31, 33, 45, 52 and 58.
It showed 98.6% efficacy against CIN 1, 96.3% efficacy against CIN 2 / 3 or AIS, 93.8% efficacy against vulvar or vaginal disease, 96.2% efficacy against persistent HPV infection six months or longer.
It also showed 96.1% efficacy against persistent HPV infection 12 months or longer, 92.6% efficacy against abnormal pap tests, 96.9% efficacy against biopsy, and 87.5% efficacy against definitive therapy, related to HPV types 31, 33, 45, 52 and 58.
The safety of Gardasil 9 was studied in six clinical trials, which enrolled more than 13,000 individuals. The most common adverse reactions found in patients administered with Gardasil 9 during the clinical studies included pain, swelling, erythema at the injection-site and headaches.
Marketing commentary
Merck plans to launch Gardasil 9 in the US market in early February 2015. It expects to receive the CDC’s Advisory Committee on Immunization Practices (ACIP) recommendation, relating to the use of product and coverage under the Vaccines for Children (VFC) programme at the February 2015 meeting.