Glybera (alipogene tiparvovec) is a gene therapy indicated for the treatment Lipoprotein Lipase Deficiency (LPLD). It was developed by Amsterdam Molecular Therapeutics (AMT), which was acquired by uniQure in April 2012.
In October 2012, uniQure received marketing authorisation approval for Glybera from the European Commission (EC) for treating patients with LPLD who have chronic and acute pancreatitis attacks. Glybera is the first gene therapy to be licensed in Europe.
Lipoprotein Lipase Deficiency (LPLD)
Lipoprotein Lipase Deficiency (LPLD) is rare genetic condition that elevates the levels of fat in the blood. The disease occurs due to alterations in LPL genes, which are responsible for breaking down of a protein-lipid complex called chylomicron. The chylomicron particles play a key role in transporting fat through the blood.
The disease results in severe and chronic pancreatitis attacks and also may cause early onset diabetes and cardiovascular complications in some patients. The symptoms of the disease include abdominal pain, and increased risk of pancreatitis.
LPLD is very rare as it occurs in one or two in every million people across the world. However, the disease is more common in some regions, including parts of Canada.
Glybera mechanism of action
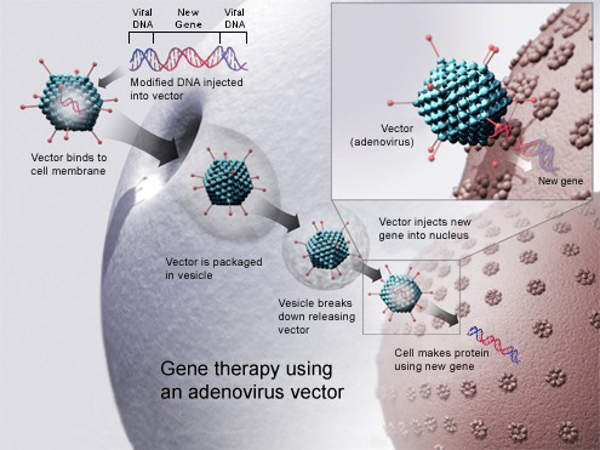
Glybera contains alipogene tiparvovec as the active substance. The drug works by breaking down the chylomicron particles present in the blood. It delivers a normal LPL gene into the body for correcting the LPL deficiency. The drug normalises the metabolism of fat in the blood and thereby prevents episodes of pancreatitis.
The drug is administered in the form of an injection into the leg muscle.
Clinical trials of Europe’s first approved gene therapy
AMT conducted a Phase II/III clinical study on Glybera between February 2009 and April 2011. It was an open label, single group assignment which enrolled five patients with LPLD. The primary outcome of the study was finding the reduction of triglyceride (TG) concentrations in 12 weeks time. The secondary outcome measures included finding the reduction in chylomicrons, determining the biological activity, and assessing the safety profile of the drug.
A 14-week Phase III open-label clinical study on Glybera was conducted in five LPLD patients from Quebec in Canada. Results of the study were announced in March 2012. The results demonstrated that one-time administration of Glybera significantly improved chylomicron metabolism after a low-fat meal.
AMT initiated another Phase II/III clinical study on Glybera in August 2007. The study is currently undergoing and expected to be completed by June 2013. It is a randomised, open label, and single group assignment study. The study enrolled 14 patients. The primary outcome measure of the study is to find reduction of fasting triglyceride (TG) concentrations. The secondary outcome measure will include finding reduction of TG concentrations, and biological activity of the transgene product.
EC approval for Glybera was based on data received from three Phase III clinical trials conducted in Canada and Netherlands. The studies enrolled a total 27 patients with LPLD.
Results from the study demonstrated that Glybera was well tolerated in all three clinical trials and no significant safety signals were detected. The patients who were administered with Glybera injection between 3 and 12 weeks evidently showed that the fat concentration in the blood was reduced. The single dose administration of Glybera reduced the frequency of acute pancreatitis.
Marketing Glybera in Europe
Glybera is currently the only gene therapy to be approved in Europe. Cerepro, another gene therapy developed by Ark Therapeutics, was rejected in the regulatory process in December 2009.
uniQure is expecting to launch Glybera in the European market by mid-2013. The drug will be available in all 27 European member states. uniQure has also applied for the approval of Glybera in the US, Canada and other countries.