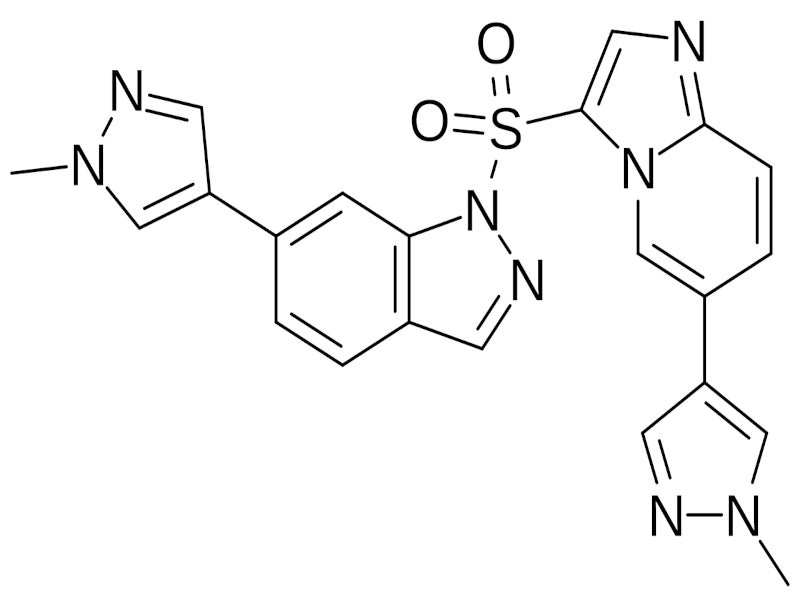
Haiyitan® (gumarontinib hydrate) is an oral mesenchymal-epithelial transition factor (MET) inhibitor, discovered and developed by Haihe Biopharma, a biotechnology company based in China.
The Category 1 innovative chemical drug was co-developed with the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, specifically for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) harbouring the MET exon 14 (METex14) skipping mutation.
Haihe Biopharma entered into an exclusive licensing agreement with Taiho Pharmaceutical, a subsidiary of Otsuka Holdings based in Japan, for the development, manufacturing, and commercialisation of gumarontinib in March 2024.
Taiho Pharmaceutical has exclusive rights for the development, manufacturing and commercialisation of the drug in Japan, Asia (excluding China), and Oceania while Haihe Biopharma will receive an upfront payment, development and sales milestones, and royalties from sales revenue, under the agreement.
In July 2024, the AmoyDx® Pan Lung Cancer PCR Panel was approved in Japan as a companion diagnostic for Haiyitan, enabling the simultaneous detection of activation alterations across 11 critical driver genes.
The panel identifies actionable mutations in seven of those genes, directly linked to 16 targeted NSCLC therapies.
Regulatory approvals for gumarontinib
The National Medical Products Administration (NMPA) granted conditional approval for Haiyitan in China for NSCLC patients with METex14 skipping mutations in March 2023.
The NMPA also designated gumarontinib as a breakthrough therapy and expedited its review under the priority review procedure.
Haihe Biopharma submitted a new drug application for gumarontinib in Japan, targeting unresectable, advanced, or recurrent NSCLC with METex14 as a potential indication in September 2023.
In November 2023, the NMPA’s Center for Drug Evaluation once again granted gumarontinib breakthrough therapy designation for the treatment of driver genes-negative, locally advanced or metastatic NSCLC with MET overexpression in patients who have progressed following prior immunotherapy and platinum-based chemotherapy.
In June 2024, the Japanese Ministry of Health, Labour and Welfare approved Haiyitan in a 50mg tablet form for patients with unresectable advanced or recurrent NSCLC with METex14 skipping mutations.
NSCLC with METex14 skipping mutations causes and symptoms
NSCLC with METex14 skipping mutations is a subtype of lung cancer caused by a genetic alteration that results in the skipping of exon 14 during the transcription of the MET gene.
This leads to an aberrant MET protein that promotes cancer cell growth and survival.
NSCLC represents approximately 85% of all lung cancers, with the proportion in Japan being 88%.
The frequency of the METex14 skipping mutation is around 3% in Japan while in the Chinese NSCLC population, it is about 1.3%. Symptoms of this NSCLC subtype include persistent cough, shortness of breath, chest pain, fatigue, weakness, and loss of appetite, among others.
Gumarontinib’s mechanism of action
Gumarontinib is a highly selective, potent, small molecule that targets and binds to the c-MET protein, disrupting c-MET-dependent signalling pathways and potentially inducing cell death in tumour cells that overexpress or harbour constitutively activated c-MET protein.
The c-MET protein is implicated in various aspects of tumour cell behaviour, including proliferation, survival, invasion, metastasis, and angiogenesis.
Gumarontinib has demonstrated excellent pharmacokinetic properties, effective and durable efficacy, and a favourable safety profile in NSCLC patients with MET alterations.
It boasts a long half-life for sustained target inhibition, low drug-drug interaction potential with fewer co-medication restrictions, and a convenient once-daily dosing regimen.
Clinical studies on gumarontinib
The GLORY Phase II clinical trial supported the approval of gumarontinib for adult patients with locally advanced or metastatic NSCLC exhibiting the METex14 skipping mutation.
The trial showcased both a tolerable safety profile and promising efficacy in both treatment-naive and previously treated patients with this mutation.
Within the GLORY study, 79 patients with a centrally confirmed METex14 skipping mutation were enrolled.
The trial’s primary endpoint, the overall objective response rate (ORR) as assessed by the Blinded Independent Review Committee, was an impressive 65.8%.
This included a 70.5% ORR in treatment-naive patients and a 60% ORR in those who had received prior treatments, observed at a 12-month follow-up.
The median progression-free survival (PFS) was reported at 8.5 months across the entire patient population.
When broken down, treatment-naive patients had a median PFS of 11.7 months, while previously treated patients had a median PFS of 7.6 months. The median overall survival stood at 17.3 months for the overall population and 16.2 months for previously treated patients.
Safety outcomes from the GLORY trial indicated that the treatment was generally well-tolerated.
Oedema emerged as the most commonly observed adverse reaction among participants.