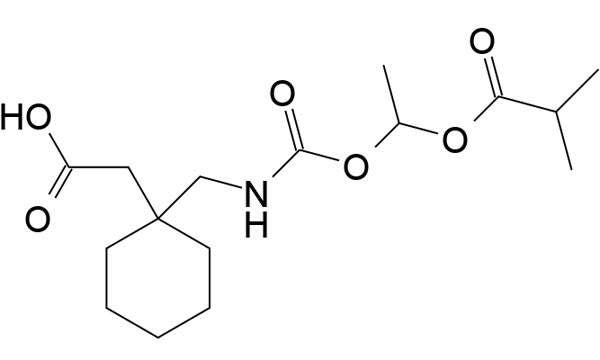
Horizant is a non-dopaminergic therapy indicated for the treatment of restless legs syndrome (RLS). It was developed by XenoPort and is marketed by GlaxoSmithKline (GSK) in the US, under a partnership agreement signed in 2007.
The US Food and Drug Administration (FDA) approved horizant in April 2011, following the New Drug Application (NDA) filed by XenoPort in January 2009. Horizant is the first drug in its class to have been approved for treating moderate-to-severe primary RLS.
In August 2011, XenoPort and GSK submitted a supplemental NDA for horizant for the treatment of postherpetic neuralgia (PHN) in adults. PHN is the pain patients experience in the area affected by shingles infection. This pain can persist for a number of years.
Symptoms of restless legs syndrome (RLS) and medication available
Restless legs syndrome, also known as Ekbom disease, is a neurological disorder characterised by an uncontrollable urge to move the legs. It is a life-long condition without any cure. The cause of RLS remains unknown. It is estimated that 1.5% to 2.7% of the population in the US suffers from RLS.
Patients suffering from RLS experience unpleasant sensations in their legs. They also experience a range of uncomfortable symptoms, including itching, tingling and burning. Most of the symptoms are predominant in the evening and early morning. These symptoms worsen with age.
Mild to moderate symptoms of RLS can be reduced or eliminated through certain lifestyle changes, such as decreased use of caffeine, alcohol and tobacco. Taking supplements for iron, folate and magnesium, massaging legs or using a heat pack may also alleviate the symptoms of RLS.
Medications used to treat RLS include dopaminergics, benzodiazepines, opioids and anticonvulsants.
Horizant’s active ingredients, mechanism of action and known side-effects
Gabapentin enacarbil, the active ingredient in horizant, gets absorbed into the body using nutrient transport mechanisms. After being absorbed, it is transformed into gabapentin which attaches to a particular kind of calcium channel.
The precise mechanism of action of horizant remains unknown. The drug does not display any affinity towards other receptors. Compared to other generic drugs available for RLS, horizant boasts several advantages. It is more effective, displays superior bioavailability and has a better dosing profile.
Horizant also has fewer side-effects. The most adverse reactions to horizant include somnolence, sedation and dizziness.
Clinical trials on the RLS / Ekbom disease treatment drug
Horizant has been tested in more than 2,300 patients who received doses ranging between 600mg and 3,600mg. The main studies were placebo-controlled and long-term follow-up trials. Ages of the patients ranged from 18 to 82 years.
Three Phase I trials were conducted to study the safety of horizant in healthy people. Two Phase II trials were conducted for horizant. One of these Phase II studies compared the safety and efficacy of horizant with placebo in 217 patients. The second compared horizant with Diphenhydramine in 130 patients.
Effectiveness of horizant was established in two Phase III trials, which compared it with a placebo.
Clinical trials for postherpetic neuralgia included four Phase II studies. Two studies tested the safety and efficacy of horizant in patients suffering from PHN. One study recruited 360 patients to compare horizant with EpiCept-NP-1 cream.
The fourth study compared horizant with a placebo and Pregabalin in 421 patients suffering from PHN.
Marketing commentary for horizant and RLS treatment drugs
Horizant has the potential to make $100m in sales annually in the US, which is expected to increase further if the drug is approved for PHN too. Although horizant offers more advantages to patients, the cost of the drug may impair its chances of being widely accepted. Generic drugs, such as Mirapex and Requip, are expected to provide tough competition for horizant.
In February 2012, XenoPort sued GSK in federal court citing contractual breach and inadequate marketing of horizant in the US. Under the agreement signed with GSK, XenoPort was to receive $312.5m in milestone payments. XenoPort, however, is yet to receive $290m.
GSK counter sued XenoPort, stating that it has held all terms of the agreement signed by the two companies. Decision of the federal court is still expected.