Iluvien is indicated for the treatment of diabetic macular oedema, an eye disease that affects the retina. The drug delivery device was licenced in 2005 by Alimera Sciences from pSivida for development as a treatment for diabetic macular oedema.
In July 2010, Alimera submitted a marketing authorisation application (MAA) to the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK for the marketing approval of Iluvien. The MHRA approved the drug in May 2012. The drug is also approved for the treatment of diabetic macular oedema in 16 other European countries, including Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Ireland, Italy, Luxembourg, the Netherlands, Norway, Poland, Portugal, Spain and Sweden.
Alimera had submitted a new drug application (NDA) to the US Food and Drug Administration (FDA) in June 2010.
The FDA declined the NDA in December 2010 and issued a complete response letter that requested further safety and efficacy data from the 36th month of the FAME study. Alimera’s NDA was based on the first 24 months of this study. The FDA also detected deficiencies in the current good manufacturing practices of Alimera’s third-party manufacturers.
In May 2011, Alimera resubmitted the NDA to the FDA. The FDA review was expected to be completed in six months. The new application included analysis of safety and efficacy data collected from the 36 month FAME trial. It also included further information about the manufacturing, packaging and sterilisation specifications of the drug.
Alimera received another complete response letter in November 2011 from the FDA for the new NDA. The letter stated that the NDA did not provide sufficient data to support further clinical trials to show that the drug is safe and effective for the proposed indication. Alimera finally obtained the FDA approval for Iluvien in September 2014. The drug was commercially launched in the US in April 2015, making it the first and only ophthalmic drug approved by the FDA for the treatment of diabetic macular oedema.
Diabetic macular oedema
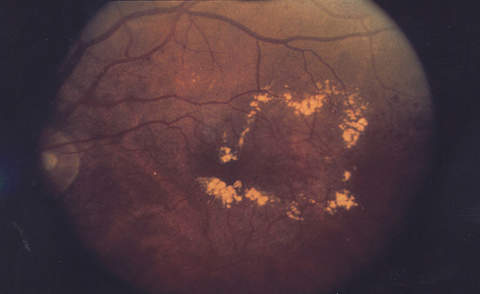
Diabetic retinopathy causes blood vessel leakage within the macula, which is a vital part of the retina responsible for central vision. When this condition happens in people with diabetes, it is called diabetic macular oedema.
Diabetic macular oedema may cause blurring of central vision or complete loss of eyesight. The onset of the disease is painless, so it may go unidentified by the patient until it manifests with complete or partial loss of vision.
According to the study by Wisconsin Epidemiologic, it was observed that over a ten-year period, more than 19% of people with diabetes were diagnosed with diabetic macular oedema.
The World Health Organisation (WHO) estimates there are more than 150 million people worldwide with diabetes, about 24 million in the US alone. All people with diabetes are prone to the development of diabetic retinopathy. In the US, diabetic macular oedema is the main cause for 12,000 to 24,000 cases of vision loss a year.
Targeting disease
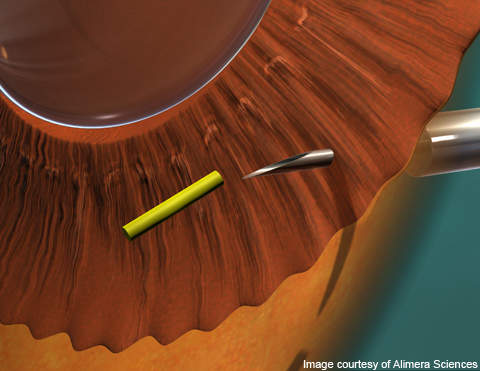
Iluvien contains fluocinolone acetonide in small microgram levels, which can be delivered directly to the patient’s eye via an intravitreal insert. The drug is inserted in the posterior of the patient’s eye through an inserter that employs a 25-gauge needle that creates a self-sealing wound. The Iluvien insert slowly releases corticosteroid into the eye for the treatment of diabetic macular oedema, and can give therapeutic effect for up to 36 months.
Clinical trials on Iluvien
Alimera conducted a phase two study on Iluvien on the first human pharmacokinetic by enrolling 37 diabetic macular oedema patients in 2008. Low doses of the drug were administered to 20 patients and a high dose was given to 17 patients. The six-month study showed the best corrected visual acuity of 15 letters or more improved by 20% in the low-dose patients and 18% in the high-dose patients.
Alimera conducted two phase three clinical trials on Iluvien between August 2007 and October 2010 in the FAME study. The research was conducted on 956 diabetic macular oedema patients from Canada, India, the US and Europe. The efficacy and safety of both low and high doses of the drug was tested for 36 months. The high dose of Iluvien administered to the patients was 0.45mg and the low dose 0.23mg a day.
The clinical trial was conducted using modified ART (all randomised and treated) methods in trials A and B. In trial A, 22.6% of the low-dose patients and 24.1% of the high-dose patients improved by 15 or more letters, compared with 12.6% of the control patients. In trial B, 29.7% of the low-dose and 29.3% of the high-dose patients improved by 15 or more letters compared with 13.3% of control patients.
The data collected during the first 24 months of the clinical trials indicated that the BCVA improved significantly, by more than 15 letters on diabetic macular oedema patients of both low-dosage and high-dosage categories.
The FAME study continued for a total of 36 months, concluding in October 2010 with the final patient visit at the three-year data point.
The FAME trial reported positive results in February 2011. The complete analysis consisted of 376 patients in ILUVIEN arm (including 190 patients in Trial A and 186 patients in Trial B) and 185 patients in the control arm. The response rates after 36 months were similar to those after 24 months.