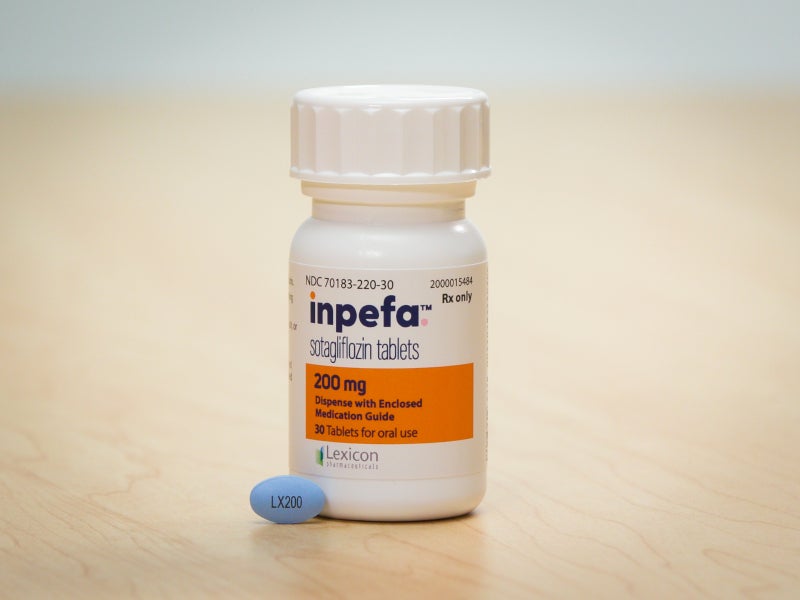
INPEFA™ (sotagliflozin) is a once-daily oral tablet indicated to reduce the risk of cardiovascular death, hospitalisation for heart failure, and urgent heart failure visit in adults with heart failure or type 2 diabetes mellitus, chronic kidney disease, and other cardiovascular risk factors.
INPEFA™ label broadly covers heart failure patients across the whole spectrum of left ventricular ejection fraction, including preserved ejection fraction and reduced ejection fraction, and for patients with or without diabetes.
The drug was developed by Lexicon Pharmaceuticals, a biopharmaceutical company based in the US, using its unique approach to gene science. Lexicon initially collaborated with Sanofi, a pharmaceutical company based in France, for the development of the drug although the partnership ended in 2019.
Brand Institute, a branding company based in the US, supported Lexicon Pharmaceuticals in the development of the brand name INPEFA™ for the product.
Sotagliflozin was approved by the US Food and Drug Administration’s (FDA) in May 2023. It is available as 200mg blue and 400mg yellow-coloured film-coated oval tablets.
Heart failure causes and symptoms
Heart failure (HF) is a progressive and debilitating condition caused by the inability of the heart to pump enough blood and oxygen to support other organs in the body.
Acute HF may develop suddenly, causing tiredness and shortness of breath, making it difficult to perform daily activities. Chronic HF causes the heart to weaken while severe HF may cause fluid build-up, which requires hospitalisation.
Multiple medical conditions, personal or family history, heart or blood vessel disorders, severe lung illnesses, HIV, Covid-19, and other infections are the risk factors for heart failure.
Smoking, drug, alcohol use, and lack of physical activity are some of the unhealthy lifestyle behaviours that contribute to HF.
HF is typically caused by underlying heart-damaging conditions, such as coronary heart disease, heart attack, inflammation, high blood pressure, cardiomyopathy, or irregular heartbeat.
In April 2023, the American College of Cardiology (ACC) highlighted the benefits of SGLT inhibitors in Guideline-Directed Medical Therapy for individuals with heart failure preserved ejection fraction.
HF remains an unmet medical need, with a prevalence of 6.7 million patients in the US in 2019, while eight million are estimated by 2030. It is the most prevalent cause of hospitalisations for adults aged 65 and above in the US, with 1.3 million hospitalisations annually.
INPEFA’s mechanism of action
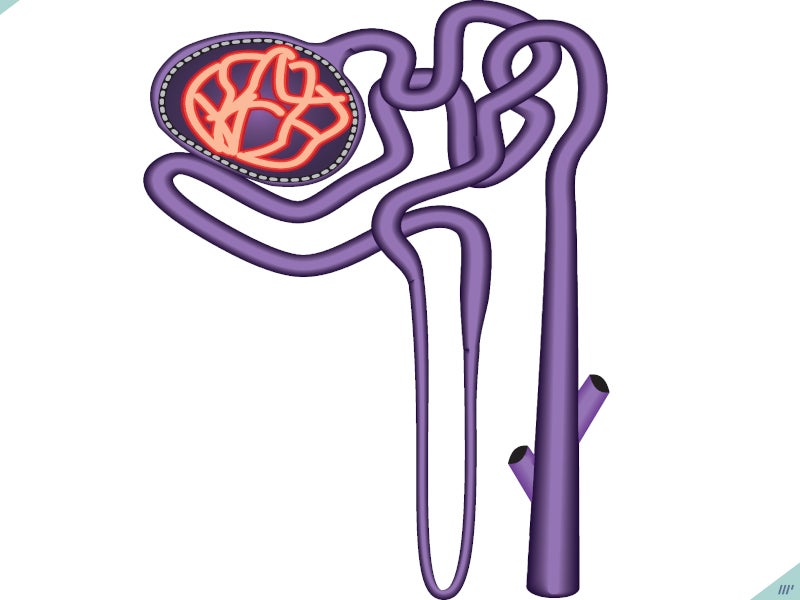
INPEFA™ is an oral dual inhibitor that blocks the activity of two proteins, SGLT 2 and SGLT1, which are responsible for glucose regulation.
SGLT2 is involved in glucose reabsorption by the kidney together with SGLT1, which absorbs glucose from the small intestine.
The American Heart Association, Heart Failure Society of America, and ACC recommend SGLT inhibitors as first-line treatment for HF.
When SGLT2 is inhibited, it decreases the reabsorption of glucose and sodium in the kidneys, potentially affecting various physiological processes, including reducing the workload on the heart before and after it pumps blood and regulating the activity of the sympathetic nervous system. On the other hand, inhibiting SGLT1 decreases the absorption of glucose and sodium in the intestines, which is likely to be a contributing factor to experiencing diarrhoea.
The specific mechanism underlying the cardiovascular advantages of sotagliflozin remains uncertain.
Clinical trial on INPEFA
The FDA approval of INPEFA™ was based on the positive outcomes of two randomised, double-blind, placebo-controlled Phase III cardiovascular outcomes studies SOLOIST-WHF and SCORED.
The clinical trials enrolled approximately 12,000 patients with HF or at risk of HF.
The primary endpoints of the studies were total occurrences of cardiovascular death, hospitalisation for HF, and urgent HF visit.
A total of 1,222 patients were randomised to receive either INPEFA™ or a placebo in the SOLOIST-WHF trial. INPEFA™ was found to be superior to placebo in terms of lowering the risk of the primary endpoint. Results show that INPEFA™ significantly reduced hospitalisations, urgent care visits, and cardiovascular deaths in patients with worsening heart failure by 33% compared to placebo.
The SCORED clinical trial included 10,584 patients, of which 5,292 were randomised to INPEFA™ and 5,292 to placebo. Results indicated a 25% risk reduction in patients treated with INPEFA™ and that the drug was superior to placebo in reducing the primary endpoint.
The most common adverse reactions observed in patients treated with INPEFA™ were urinary tract infection, volume depletion, diarrhoea, and hypoglycaemia.