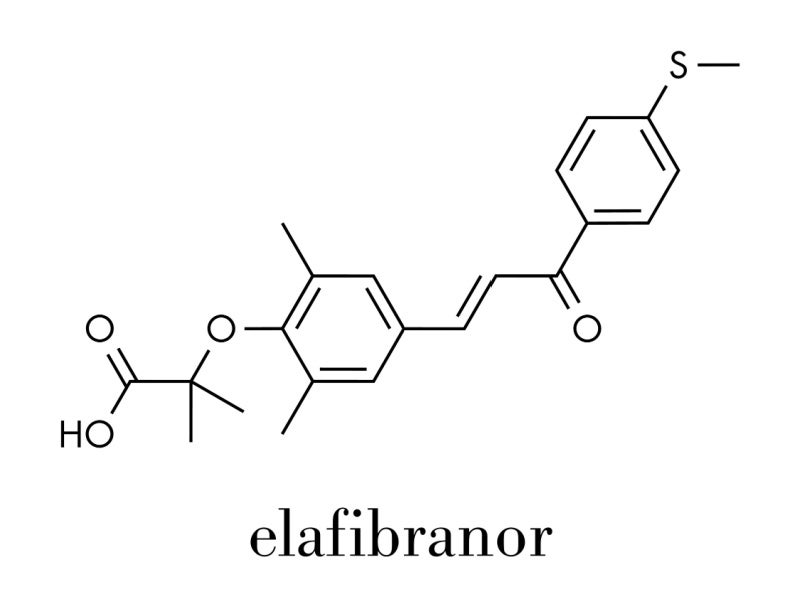
Iqirvo® (elafibranor) is a first-in-class peroxisome proliferator-activated receptor (PPAR) agonist indicated for the treatment of primary biliary cholangitis (PBC) in adults in combination with ursodeoxycholic acid (UDCA) for patients who do not respond adequately to UDCA, or as the sole therapy for those intolerant to UDCA.
Elafibranor was originally developed by GENFIT, a biopharmaceutical company based in France.
In December 2021, Ipsen, another French biopharmaceutical company, entered into an exclusive licence agreement with GENFIT and secured the rights to develop, manufacture, and commercialise Iqirvo globally, except China, Hong Kong, Taiwan, and Macau where Terns Pharmaceuticals, a US-based pharmaceutical company holds the rights for the treatment of non-alcoholic steatohepatitis and PBC.
Iqirvo is available for oral administration as round, orange-coloured, film-coated tablets in a dosage strength of 80mg.
Regulatory approvals for Iqirvo
In June 2024, the US Food and Drug Administration (FDA) granted accelerated approval to Iqirvo for the treatment of PBC following the FDA’s acceptance of a New Drug Application for priority review in December 2023, submitted by Ipsen in October 2023.
The approval was based on the reduction of ALP levels, a biochemical marker used as a surrogate endpoint in PBC clinical trials.
The FDA awarded breakthrough therapy designation to Iqirvo in 2019.
In September 2024, the EU approved the use of Iqirvo, granting it conditional marketing authorisation. Earlier, in July 2019, the European Medicines Agency granted orphan designation to the drug.
Currently, Iqirvo is under review by the UK Medicines and Healthcare Products Regulatory Agency.
Primary biliary cholangitis causes and symptoms
PBC, formerly known as primary biliary cirrhosis, is a chronic autoimmune disease characterised by an impaired or obstructed bile flow, known as cholestasis.
In PBC, inflammation leads to the gradual destruction of small bile ducts within the liver, resulting in the accumulation of bile and potentially severe liver complications if not treated.
In the US, the condition affects approximately 100,000 individuals, predominantly middle-aged women.
The main causes of PBC are genetic disorders, infections, cigarette smoking, chemical exposure, and other environmental factors.
Although many patients exhibit no symptoms upon initial diagnosis, some symptoms can indicate the presence of the disease such as persistent fatigue and pruritus, a chronic itching sensation.
Iqirvo’s mechanism of action
Elafibranor and its principal active metabolite, GFT1007 activate PPAR-alpha (α), PPAR-gamma, and PPAR-delta (δ), leading to cholesterol reduction, increased insulin sensitivity, and improved glucose and fatty acid metabolism.
The precise mechanism by which elafibranor benefits PBC patients is not fully understood. Its pharmacological actions that may be pertinent to its therapeutic impact involve the suppression of bile acid production by stimulating PPARα and PPARδ.
Notably, the activity of cholesterol 7 alpha-hydroxylase-1, the enzyme responsible for the conversion of cholesterol into bile acids is regulated upon PPARδ activation. The regulation is influenced by a protein called fibroblast growth factor 21.
Clinical trials on Iqirvo
The accelerated approval of Iqirvo was based on the outcomes of the Phase III ELATIVE clinical trial.
ELATIVE is a multi-centre, randomised, double-blind, placebo-controlled study that assessed the efficacy and safety of Iqirvo in combination with UDCA versus a placebo with UDCA.
Approximately 161 participants were randomised in a 2:1 ratio to receive either Iqirvo or a placebo, once daily for at least 52 weeks.
The primary endpoint, a biochemical response at week 52, was achieved by 51% of patients treated with Iqirvo, compared to 4% in the placebo group.
Also, studies have shown that the reductions in alkaline phosphatase levels from baseline were sustained until week 52 in the Iqirvo cohort, with a rapid response evident as early as week 4. Iqirvo treatment led to significantly enhanced improvements in cholestasis biochemical markers compared to placebo.
The most frequently reported adverse events among patients were musculoskeletal issues, obesity, abdominal discomfort, diarrhoea, nausea, and vomiting.
The company is conducting the confirmatory ELFIDENCE trial to support the continued approval of Iqirvo.