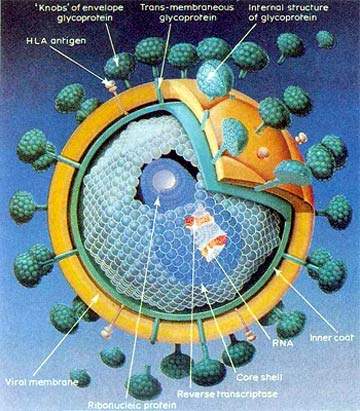
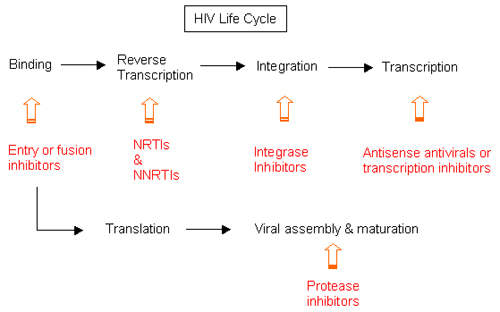
Under development by Merck & Co, Isentress (raltegravir) is an experimental drug indicated for the treatment of human immunodeficiency virus (HIV) infection. It is a member of a new class of oral HIV drugs called integrase inhibitors, several of which are currently in development.
In October 2007, the drug received FDA approval for use in combination with other anti-retroviral therapy for the treatment of HIV infection in treatment-experienced patients with ongoing viral replication despite existing therapy.
Merck’s Isentress (raltegravir) is the first integrase inhibitor to reach the US market. In Europe, the drug received a positive recommendation from the EMEA’s Committee for Medicinal Products for Human Use (CHMP) in November 2007.
In January 2008, Isentress was approved by the EU for use along with other antiretrovirals in patients whose existing combination is failing to control viral load. In September 2009, the EU approved an expanded licence to Isentress for use along with other antiretroviral medicinal drugs for HIV-1 infection indication in adult patients, first time users as well as those who are treatment-experienced.
Integrase inhibitors – a new class of HIV drugs
First-line therapy for HIV infection typically entails the use of a combination regimen of highly active anti-retroviral drugs, ideally initiated before a patient’s CD4 cell count falls below 350–300 cells/ml.
Highly active anti-retroviral therapy, or HAART, usually includes combinations of thymidine nucleoside analogues with a non-thymidine nucleoside analogue. A protease inhibitor may also be included in the regimen.
While similar to established HIV drugs in targeting HIV after it has invaded the host’s immune cells, integrase inhibitors differ in that they act on the HIV integrase enzyme that is responsible for insertion of viral DNA into human DNA – an early stage in the HIV lifecycle. By blocking the activity of HIV integrase enzyme, this new class of anti-retroviral drugs stops the virus from replicating and infecting new cells.
Isentress (raltegravir) suppresses viral load
US recommendations to approve Isentress (raltegravir) followed an analysis of efficacy and safety data from studies in which it was used in combination with optimised background therapy (OBT) in treatment-experienced HIV-infected patients who had failed anti-retroviral therapies, and become resistant to at least one drug in each of three classes of oral drugs.
Interim data from the phase III Benchmrk 1 and 2 studies in treatment-experienced patients showed that a greater number of patients in the Isentress treatment arm achieved a viral load below 400 copies/ml compared with those on OBT plus placebo (79% vs. 43% respectively).
These data in a difficult-to-treat HIV patient population compare very favourably with rates of response (<400 copies/ml) seen in treatment-naïve patients receiving HAART, which are typically in the range 60–80%.
In addition to trials in treatment-experienced patients, Merck is also conducting phase III trials to assess the efficacy and safety of Isentress in previously untreated HIV-infected patients. Available trial data have shown that when used in combination with tenofovir and lamivudine, Isentress achieved comparable reductions in viral load to combined treatment with tenofovir, lamivudine and efavirenz.
Over 48 weeks, 83–88% of treatment-naïve patients in the raltegravir treatment arm had HIV RNA reductions of <50 copies/ml (undetectable levels), compared with 87% of those in the comparator arm. Reductions in viral load were accompanied by improvements in CD4 cell counts.
Expanding treatment options for HIV patients
Although there are now a large number of approved drugs for treatment of HIV, the development of drug resistance can severely limit their clinical effectiveness.
There remains an urgent need for new classes of drugs to combat HIV infection in the growing number of people who have exhausted standard regimens.
Clinical data from trials carried out on Isentress suggest that its addition to OBT for HIV can greatly improve and sustain viral suppression in treatment-experienced patients. Potential exists not only to incorporate integrase inhibitors such as Isentress (raltegravir) into established regimens but also to explore combinations of new classes of anti-retroviral drugs.
Investigations are already underway to assess the effects of combining Isentress (raltegravir) with Pfizer’s novel CCR5 inhibitor, maraviroc, which has recently been approved as a treatment for HIV.
Marketing commentary
Since its discovery in the early 1980s, infection with HIV has reached epidemic proportions, especially in developing countries. Estimates suggest that 42 million people are living with HIV/AIDS, of whom around 3 million or more die each year.
The advent of HAART in the 1990s had a major impact on the treatment of HIV. While unable to eliminate the virus from the body, the use of combinations of drugs from different classes proved effective in reducing symptoms, improving immune status, and delaying the onset of AIDS.
However, over time some infected individuals develop drug resistance and become unresponsive to all the drugs in a particular class. According to the WHO, as many as 20% of people in North America and Europe are infected with strains of HIV resistant to at least one class of anti-retroviral drug. Isentress attacks HIV via an alternative mechanism to established classes of drugs and thus holds promise for those patients who have failed established treatment options.