Istaroxime is an investigational drug for acute decompensated heart failure under development by Debiopharm of Switzerland. It was originally identified and developed by sigma-tau Industrie Farmaceutiche Riunite SpA (sigma-tau) of Italy, from which Debiopharm acquired the right to develop and commercialise istaroxime worldwide, except in Italy. These rights also extend to istaroxime follow-on compounds.
Istaroxime is still in early-stage development, having been evaluated in phase II trials in patients with decompensated heart failure.
THE BURDEN OF CHF
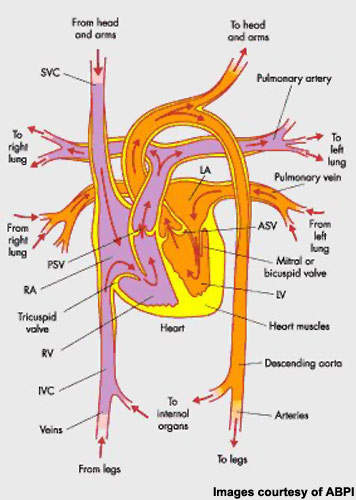
Congestive heart failure (CHF), a condition in which the pumping action of the ventricle is impaired, is associated with significant morbidity and mortality. For patients diagnosed with severe CHF, one-year mortality approaches 60%. Even for patients with mild to moderate CHF, one-year mortality is up to 30%. In patients with CHF, acute decompensation is a major cause of hospitalisation and of costs associated with treatment.
Figures from the US suggest that some five million adults have CHF, while about ten million people in Europe have CHF. These figures are projected to grow as the proportion of elderly in the population increases. Indeed, CHF affects about 10% of the elderly population. In the US, about 250,000 deaths a year are attributable to CHF.
CHF can be related to systolic or diastolic, of which ventricular systolic dysfunction is most common, accounting for about 60% of all cases of CHF.
ISTAROXIME – FIRST-IN-CLASS LUSO-INOTROPIC AGENT
In CHF, impaired ventricular function leads to fluid retention as blood accumulates in the vessels surrounding the lungs, producing the characteristic symptoms of fatigue, breathlessness (dyspnoea) and oedema. Treatment is designed to address the underlying cause of CHF, improve symptoms and quality of life.
Stabilisation and improvement of cardiac haemodynamics is an important goal of therapy. Inotropic support of depressed ventricular function is important in this regard, with intravenous inotropes the mainstay therapy in patients with an acute exacerbation of heart failure when haemodynamic support is needed.
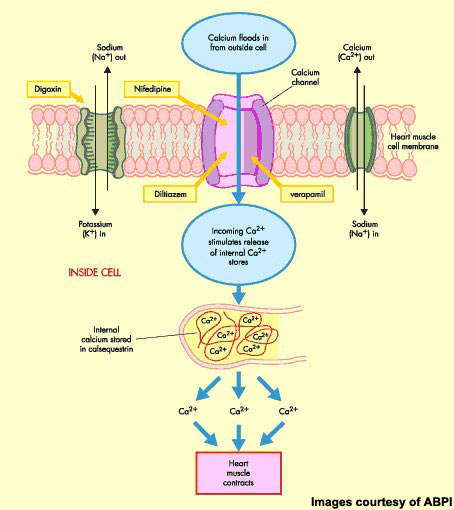
Istaroxime is a first-in-class luso-inotropic agent that possesses a dual mode of action, combining inotropic (stimulation of myocardial contractility during systole) and lusitropic (improvement of diastolic relaxation) effects. It modulates calcium cycling through inhibition of the Na+K+-ATPase and activation of the sarcoplasmic reticulum Ca-ATPase, SERCA2a. Na+/K+-ATPase inhibition augments cytoplasmic calcium levels, promotes cardiac myocyte contractility and strengthens the force of the heartbeat, while SERCA2a activation promotes the reuptake of cytoplasmic calcium, allowing the myocytes to relax again and blood to re-enter the heart.
Preclinical studies showed that istaroxime increases myocardial contractility without increasing heart rate and oxygen consumption. It also improved ventricular relaxation and lowered preload.
EFFICACY IN PHASE II TRIALS
Early clinical trials in CHF patients with left ventricular dysfunction indicated that treatment with istaroxime produced dose-dependent increases in recorded myocardial contractility without increases in heart rate or signs of arrhythmogenesis. Safety studies also showed it did not induce chronotropic response or prolong QT intervals.
In a subsequent phase II trial in patients with decompensated heart failure, treatment with istaroxime was associated with a rapid improvement in cardiac haemodynamics and diastolic function without adverse neurohormonal or renal effects.
This randomised, double-blind, placebo-controlled, multicentre trial enrolled 120 patients with worsening heart failure: left ventricular ejection fraction was below 35% and a pulmonary capillary wedge pressure above 20 mmHg.
Compared with placebo recipients, patients in the istaroxime treatment arms demonstrated a statistically significant decrease of pulmonary wedge pressure (p<0.03 for all doses), a reduction of left ventricular diastolic volume (p=0.02 for the highest dose) and an increase in cardiac index (p=0.04 for the highest dose).
MARKETING COMMENTARY
Intravenous inotropes are used in patients with an acute exacerbation of heart failure when haemodynamic support is needed. Currently available agents, such as digoxin, suffer a number of significant drawbacks including risks of increased heart rate and hypotension as well as atrial and ventricular arrhythmias.
Although istaroxime is still in early-stage development, results released to date suggest that it may offer improved systolic and diastolic function without causing significant arrhythmias or ischemia or increasing myocardial oxygen consumption. This suggests it may have an improved safety margin over other inotropic agents but clearly more clinical data is needed.