Januvia (sitagliptin phosphate) is an antihyperglycaemic drug containing an orally active inhibitor of the dipeptidyl peptidase-IV (DPP-IV) enzyme. Developed by Merck Sharp & Dohme (MSD), a UK subsidiary of Merck & Co, sitagliptin is used for treating type 2 diabetes mellitus. The drug has proved effective in lowering blood sugar levels of diabetes patients when taken alone or in combination with other oral diabetes medications such as metformin and thiazolidinedione.
Sitagliptin was approved by the US Food and Drug Administration (FDA) in October 2006 and is marketed under the brand name Januvia in the US. Sitagliptin in combination with metformin was approved by the FDA in March 2007 and is marketed as Janumet in the US. In the EU, Januvia was approved in April 2007 and Janumet was approved in July 2008.
In June 2009, Merck announced that two new investigational studies that evaluated the efficacy of Januvia showed it substantially improved blood sugar control. One study assessed the efficacy of Januvia in combination with insulin with or without metformin and the second study assessed the effectiveness of Januvia with pioglitazone. New drug applications to use Januvia in these combinations and Janumet in combination with insulin have been accepted by the FDA and are under review. If the applications are approved, Januvia and Janumet will provide more options for patients suffering with type 2 diabetes.
Merck is conducting a study on cardiovascular outcomes with Januvia. The study will enroll about 14,000 patients over two years with type 2 diabetes and pre-existing cardiovascular disease. The first patient for the study was enrolled in December 2008. The study is a multinational, placebo-controlled, double-blind, randomised, parallel-group clinical trial with a follow up of four to five years.
Diabetes mellitus
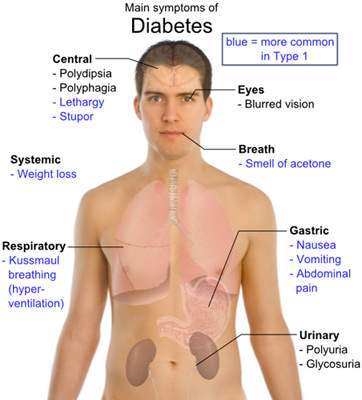
Diabetes mellitus or type 2 diabetes is the most common form of the disease, accounting for about 90–95% of all diagnosed cases of diabetes worldwide. As people are leading a more sedentary lifestyle, incidence of diabetes is expected to increase.
In type 2 diabetes, the body does not produce adequate insulin or the cells ignore the insulin. Insulin is essential to take sugar, the main fuel for cells, from the blood into the cells. High blood sugar levels can give rise to serious complications such as heart diseases, blindness, and damage of nerves and kidneys.
Type 2 diabetes is complicated to treat in elderly patients as advanced age increases the risk of hypoglycaemia. Attaining target glycaemic levels and avoiding low blood sugar is a challenge in such cases. Symptoms of hypoglycaemia include shakiness, dizziness, sweating, hunger, headache, pale skin colour, seizure, confusion and unconsciousness.
Three-way action of Januvia
Januvia lowers blood sugar in three ways: by increasing the insulin produced in the pancreas, addressing insulin resistance and decreasing the sugar produced in the liver.
Januvia enhances a natural body system known as the incretin system which helps in regulating blood sugar by increasing levels of active glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) hormones.
Januvia contains phosphate which builds a peptide chain with glucose, thereby reducing the activity of hormones and formation of glucagons. As a result, insulin levels increase to the quantity required by a patient suffering from type 2 diabetes. It also helps in production and release of insulin until the glucose level in the blood is restored to normal.
Since Januvia is an enzyme-inhibiting drug, it has only a few side effects such as weight gain (less risk), hypoglycaemia (less risk), sore throat, upper respiratory infection and headache.
Clinical trials
Clinical trials for sitagliptin conducted by Merck include 55 studies that have been completed or are underway. About 3,800 patients with type 2 diabetes were involved in six double-blind, placebo-controlled clinical safety and efficacy studies. The studies assessed the impact of sitagliptin on glycaemic control. Januvia produced significant improvements in haemoglobin A1C, fasting plasma glucose (FPG) and two hour post-prandial glucose (PPG) compared to placebo.
To evaluate the efficacy and safety of Januvia monotherapy, 1,262 patients with type 2 diabetes were enrolled in two double-blind, placebo-controlled studies. One study was of 18-week duration and the other of 24-week duration. The overall impact of Januvia was similar to placebo.
To test the efficacy of Januvia in combination with metformin, 701 patients with type 2 diabetes were enrolled in a 24-week, randomised, double-blind, placebo-controlled study. The study revealed significant improvements in A1C, FPG and two-hour PPG compared to placebo when Januvia was used in combination with metformin.
In November 2008, Merck presented new study data at the 61st Annual Scientific Meeting of the Gerontological Society of America. The study showed significantly reduced blood sugar levels without any incidence of hypoglycaemia in 206 patients aged between 65 and 96. The study was a 24-week randomised, double-blind, placebo-controlled trial.
Marketing commentary
Diabetes affects about 180 million people across the world and the figure is expected to increase to 366 million by 2030.
As diabetes is one of the world’s fastest-growing diseases, worldwide sales of diabetes drugs are expected to increase by 33% to $22bn by 2016.
Compared to other drugs that treat type 2 diabetes, Januvia has very few side affects. Other drugs have been reported to have harmful side-effects such as heart attacks. Januvia, therefore, registered robust sales for Merck since its approval. In the first quarter of 2009, Januvia recorded sales worth $411m.