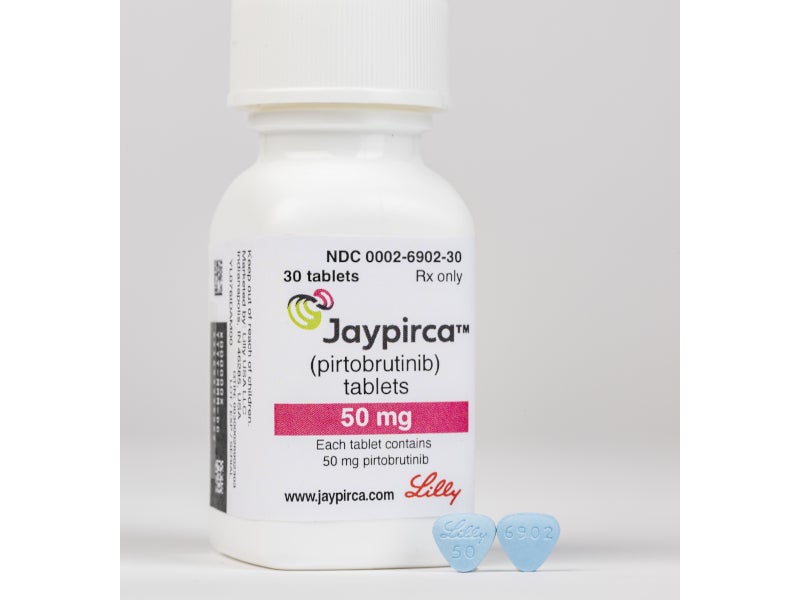
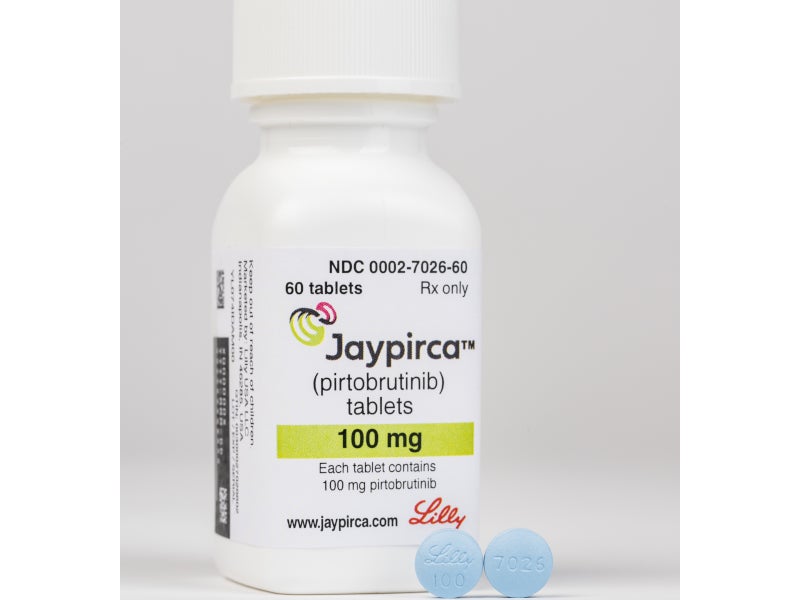
Jaypirca™ (pirtobrutinib), formerly known as LOXO-305, is a non-covalent Bruton’s tyrosine kinase (BTK) inhibitor for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (r/rMCL), who have previously been treated with a covalent BTK inhibitor.
Developed by Eli Lilly and Company’s Loxo@Lilly oncology unit, Jaypirca is available as 50mg (arc-triangle shaped) and 100mg (round) blue, film-coated tablets for oral administration.
Regulatory approvals for Jaypirca
Eli Lilly began sending submissions to the US Food and Drug Administration (FDA) for pirtobrutinib in December 2021.
Pirtobrutinib was granted accelerated approval by the FDA for the treatment of adult patients with r/r MCL in January 2023.
Mantle cell lymphoma causes and symptoms
MCL is a rare blood cancer and a subtype of a B-cell lymphatic cancer called non-Hodgkin’s lymphoma (NHL). It originates in the B lymphocytes located in the mantle zone region of the lymph node.
As the cancer grows, it might spread to other sites including the bone marrow, spleen, liver, or digestive tract. MCL affects approximately one out of 200,000 individuals a year globally. MCL is three times more common in males than females. People aged over 60 years are more susceptible to the disease. The median overall survival for patients with MCL is between four and five years.
The symptoms of MCL might vary depending on the intensity and location of the disease. It may include enlarged lymph nodes, unexplained fevers, night sweats, decreased appetite, unexpected weight loss, headaches, and weakness/fatigue.
Jaypirca’s mechanism of action
Jaypirca is a small-molecule, highly selective, ATP-competitive inhibitor of BTK, a signalling protein responsible for the development and maturation of B cells.
BTK signalling activates pathways necessary for B-cell proliferation, trafficking, chemotaxis, and adhesion in B cells. Jaypirca binds to both wild-type BTK and BTK with C481 mutations, inhibiting BTK kinase activity.
Jaypirca inhibited BTK-mediated B-cell CD69 expression and malignant B-cell proliferation in non-clinical studies. It showed dose-dependent anti-tumour activity in BTK wild-type and BTK C481S mutant mice xenograft models.
Clinical trials on Jaypirca
The FDA approval of Jaypirca was based on the phase I/II, open-label, single-arm and multi-centre clinical trial BRUIN.
The trial evaluated the efficacy of Jayprica as a monotherapy in 120 patients with r/r MCL who have been previously treated with a covalent BTK inhibitor such as ibrutinib, acalabrutinib, or zanubrutinib.
All the patients had a median of three prior lines of treatment, with 93% having two or more prior lines. Approximately 83% of the patients had stopped using their last BTK inhibitor, owing to refractory or progressive disease. Others discontinued their last BTK inhibitor due to toxicity and other reasons.
The patients were administered a 200mg dose of pirtobrutinib once daily and maintained until disease progression or unacceptable toxicity.
The primary outcome measure was the overall response rate (ORR) and duration of response (DOR) assessed by an independent review committee (IRC), based on the Lugano criteria (2014).
In the trial, 50% of the patients achieved the ORR, with 13% of them achieving a complete response. The estimated median DOR was 8.3 months.
The most common adverse reactions observed in patients during the clinical trial were fatigue, musculoskeletal pain, diarrhoea, oedema, dyspnoea, pneumonia, and bruises.