Kengreal (cangrelor) is the first and only intravenous anti-platelet agent indicated as an adjunct to percutaneous coronary intervention (PCI) for reducing thrombotic events. The drug was discovered and is being developed by The Medicines Company.
Kengreal was approved by the US Food and Drug Administration (FDA) in June 2015 as an adjunctive therapy to PCI for reducing the risk of periprocedural thrombotic events, such as myocardial infarction (MI), stent thrombosis (ST), and ischemia-driven revascularisation.
The drug is indicated for patients who have never been treated with a P2Y12 inhibitor and glycoprotein IIb / IIIa inhibitor (GPI).
The approval follows the Cardiovascular and Renal Drugs Advisory Committee’s (CRDAC) recommendation in April 2015 to approve. The drug will be available in the US by July 2015.
Cangrelor was approved in the European Union under the trade name, Kengrexal. The European Commission granted marketing authorisation for the drug in March 2015 following the positive opinions issued by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) in January. The marketing authorisation is valid in the 31 countries of the European Economic Area (EEA).
Percutaneous coronary intervention (PCI) – need for effective adjunctive therapies
Brilinta is an anti-platelet drug which decreases the platelet aggregation and prevents blood clots.
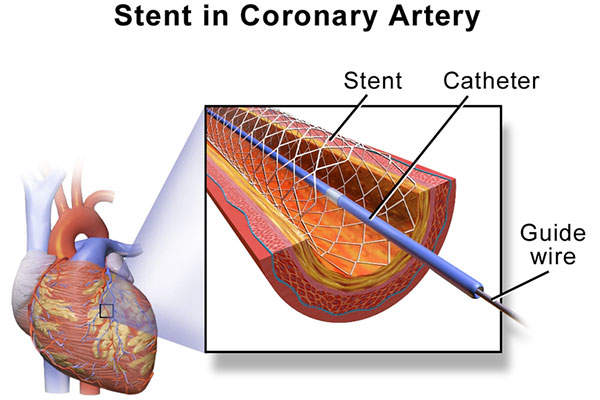
Percutaneous coronary intervention, popularly known as coronary angioplasty, is a non-surgical technique to treat narrowed arteries in coronary heart disease patients.
PCI along with stent implantation is a widely-used practice to reduce the risk of death or heart attack in patients with acute coronary syndrome and to reduce the pain associated with angina in patients with stable angina.
It was estimated that more than 700,000 PCIs in the US and approximately one million PCIs in Europe are done a year, which require effective anti-thrombin and anti-platelet therapies.
Despite advances in the adjunctive therapies, thrombotic complications, including blood clot, remain to be a major concern during PCI. Available anti-platelet therapies such as oral P2Y12 inhibitors and intravenous glycoprotein IIb/IIIa inhibitors reduce the risk of ischemic events, but have numerous limitations including a delayed onset of action and delayed reversal.
This led to the need of a novel anti-platelet agent well-suited for contemporary practice in the catheterisation (cath) lab.
Cangrelor’s mechanism of action
Cangrelor is a direct-acting P2Y12 platelet receptor antagonist that blocks adenosine diphosphate (ADP)-induced platelet activation. It binds selectively and reversibly to the P2Y12 receptor to prevent further signalling and platelet activation.
The drug shows immediate effect, which can be maintained with a continuous infusion. The platelets restore to normal function within an hour of ceasing the infusion.
Clinical trials on Cangrelor
The FDA and EMA approvals were based on the results from a phase III clinical study named Champion Phoenix, conducted to determine the safety and efficacy of the drug.
The double-blind, placebo-controlled trial enrolled 11,145 patients, who were undergoing PCI and were receiving recommended therapy of congrelor or a dose of clopidogrel.
The study compared cangrelor to clopidogrel, both given in combination with aspirin and other standard therapy. The subjects were randomised to receive an infusion of cangrelor or matching placebo and the first set of capsules containing either 600mg or 300mg of clopidogrel or matching placebo. At the end of the infusion, patients received a second set of capsules containing either 600mg of clopidogrel (cangrelor group) or matching placebo (clopidogrel group).
The primary efficacy end point of the study was composite rate of death from any cause, myocardial infarction (MI), ischemia-driven revascularisation (IDR), or stent thrombosis (ST) at 48 hours after randomisation. The key secondary end point was stent thrombosis at 48 hours and the primary safety end point was severe bleeding at 48 hours.
The results demonstrated that cangrelor significantly reduced the primary composite endpoint of all-cause mortality, MI, IDR and ST compared to clopidogrel at 48 hours, in 30 days.
The most common adverse reactions observed during the study were bleeding and dyspnea.