Tykerb (lapatinib ditosylate) is an epidermal growth factor receptor (EGFR) and ErbB-2 (Her2/neu) dual tyrosine kinase inhibitor, under development by GlaxoSmithKline as a treatment for solid tumours such as breast and lung cancer. This novel investigational agent has attracted considerable interest, as it appears to arrest the development of breast cancer in some patients with metastatic, treatment-refractory disease.
Following submissions for regulatory approval in 2006, in 2007 both the US FDA and EMEA approved Tykerb/Tyverb (lapatinib ditosylate) for patients with metastatic (advanced) HER2 positive breast cancer previously treated with other anticancer drugs including trastuzumab (Herceptin). It is licensed for use in these patients in combination with capecitabine.
Tyrosine kinase inhibitors (TKI) represent a growing class of anti-cancer agents
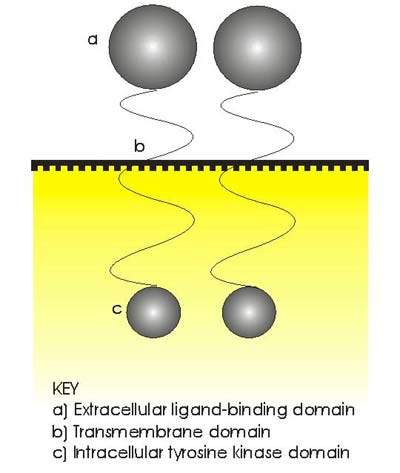
Protein tyrosine kinases are enzymes that provide a central switch mechanism in cellular signal transduction pathways. As such they are involved in many cellular processes such as cell proliferation, metabolism, survival and apoptosis. Several protein tyrosine kinases are known to be activated in cancer cells and to drive tumour growth and progression.
Blocking tyrosine kinase activity therefore represents a rational approach to cancer therapy. Therapeutic strategies include blocking kinase-substrate interaction, inhibiting the enzyme’s adenosine triphosphate (ATP) binding site and blocking extracellular tyrosine kinase receptors on tumour cells. Already several TKIs have been approved as anti-cancer agents.
The erbB or HER family of transmembrane tyrosine kinase receptors, especially receptors erbB1 (or EGFR) and erbB2 (or Her2/neu), has been identified as an important therapeutic target in a number of cancers. Her2/neu, for example, is overexpressed in around 20% to 30% of patients with aggressive breast cancer, while EGFR is overexpressed in several solid tumours.
GSK’s Tykerb (lapatinib ditosylate) is a small molecule, orally-active TKI that targets both erbB receptors.
Its dual mode of action distinguishes it from existing TKIs such as gefitinib (Iressa) and trastuzumab (Herceptin), which are single EGFR and Her2/neu receptor inhibitors respectively. It is hoped that dual TKIs may help to address the problem of drug resistance that can arise following treatment with single receptor inhibitors.
Clinical data supports use in breast cancer patients
A growing body of clinical data is now available to support the clinical effectiveness of GSK’s Tykerb (lapatinib ditosylate) in advanced breast cancer, including new data on its use in combination with other drugs.
Tykerb (lapatinib ditosylate) in combination with pazopanib, an investigational TKI and angiogenesis inhibitor, was just one example of new data presented at the 2008 meeting of the American Society of Clinical Oncology (ASCO) meeting in Chicago.
In the first phase II trial to investigate the effect of combining these two drugs for first-line use in HER2 positive breast cancer, no significant difference was observed in progressive disease rate at week 12 (primary endpoint) between combination therapy and lapatinib alone. However, response rates were higher in the combination treatment arm, which suggest that further studies of first-line lapatinib and pazopanib are worthwhile.
A second phase II trial investigated the effects of combining labpatinib with the angiogenesis inhibitor Avastin (bevacizumab). Again there was evidence to suggest that in heavily pre-treated patients, combination therapy can be beneficial. In this trial, combination therapy conferred a 34.4% clinical benefit at Week 24 (complete response/partial response/ stable disease), while 62.5% of patients were progression free at Week 12.
There is also encouraging evidence to suggest that Tykerb (lapatinib ditosylate) may have efficacy in inflammatory breast cancer, a rare but highly aggressive form of the disease that has a particularly poor prognosis. In the largest prospective clinical trial in relapsed/refractory HER2 positive inflammatory breast cancer to date, lapatinib demonstrated efficacy in 126 patients refractory to treatment with anthracyclines and taxanes. With a response rate approaching 40%, median duration of response was 20.9 weeks and overall progression-free survival 14.6 weeks.
Based on the available data, once-daily oral Tykerb (lapatinib ditosylate) appears generally well tolerated with rash, fatigue and diarrhoea – the most common treatment-related adverse events to be reported among patients treated to date.
New therapeutic paradigms for patients with solid tumours
The recent introduction of several new classes of anti-cancer drugs, such as the TKIs, has opened the door to new approaches to cancer treatment, in which the goals of therapy are to halt disease progression, ameliorate symptoms and improve patient quality of life.
Often described as targeted cancer therapies, these new agents have the potential to prolong life without the serious side effects associated with cytotoxic drugs that have traditionally underpinned the treatment of most solid tumours. Many of the new targeted anti-cancer agents possess cytostatic rather than cytotoxic properties. Increasingly, oncologists envisage a time when cancers will be turned from life-threatening diseases into manageable chronic conditions.
Marketing commentary
Although there have been major advances in the treatment of breast cancer in the last 10–15 years, it remains a disease for which new treatments are still urgently needed. This is especially true for patients whose disease recurs and progresses following treatment.
Among currently available TKIs, trastuzumab (Herceptin) has made a significant contribution to the treatment of metastatic breast cancer. However, eventually most patients with metastatic breast cancer receiving trastuzumab-containing regimens experience disease progression, with salvage chemotherapy effective in about 10% of cases.
Approval of Tykerb/Tyverb (lapatinib ditosylate) in HER2 positive metastatic breast cancer offers an important advance for patients with this type of breast cancer who fail to benefit from continued trastuzumab-based therapy.