Developed by the German pharmaceutical company, Merckle GmbH, together with EuroAlliance partners Alfa Wassermann and Lacer, licofelone (ML3000) is a dual COX/LOX inhibitor and the first member of this new class of analgesic and anti-inflammatory drugs.
It is currently under evaluation as a treatment for osteoarthritis (OA), the most common form of arthritis. Although phase III trials have been successfully completed in OA patients no dates for regulatory submission have yet been given.
Dual COX/LOX inhibition for relief of pain and inflammation
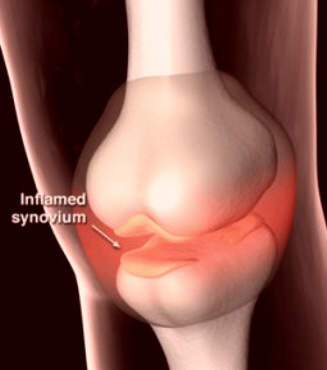
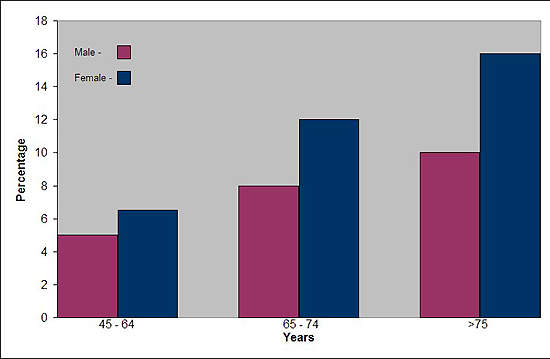
Affecting about 10% of adults in the world’s industrialised countries, OA is a common degenerative disease of the joints characterised by the breakdown of cartilage and the proliferation of new bone and connective tissue. Drugs that can modify disease progression represent the ultimate goal of treatment but are not clinically available.
As the search for disease-modifying drugs continues, treatment is currently based on symptomatic relief of pain and inflammation associated with OA.
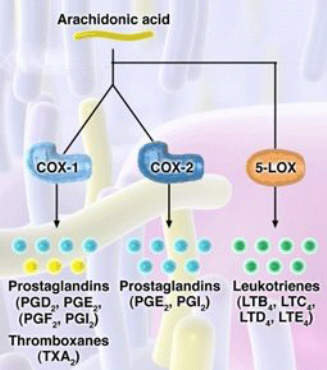
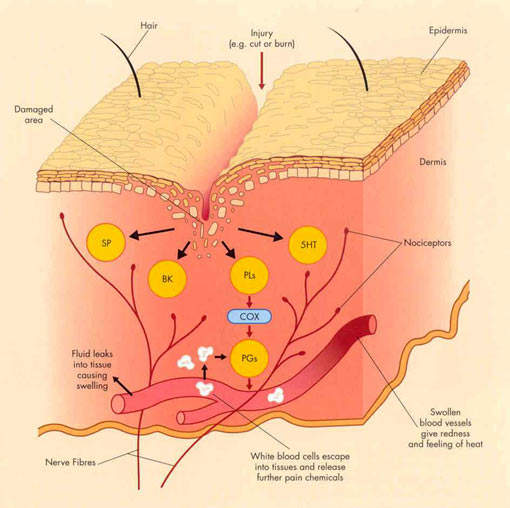
For years non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen and naproxen, have underpinned symptomatic relief of OA. NSAIDs work by inhibiting the cyclooxygenase (COX) enzymes COX-1 and COX-2, so preventing the formation of inflammatory prostaglandins from metabolism of arachidonic acid.
However, by inhibiting COX-1 they can also cause serious gastrointestinal (GI) side effects and adversely affect platelet function.
Increasingly, conventional NSAIDs are being replaced by
COX-2 specific inhibitors, such as celecoxib, for symptomatic relief of OA. At therapeutic concentrations these drugs inhibit COX-2, which is associated with tissue injury, but spare COX-1 and so cause less GI toxicity.
Merckle’s licofelone differs from both conventional NSAIDs and COX-2 specific inhibitors in that it inhibits not only COX but also 5-lipoxygenase (5-LOX), an enzyme associated with the production of pro-inflammatory and gastrotoxic leukotrienes. Evidence suggests that NSAID-induced GI toxicity may involve shunting arachidonic acid metabolism to the 5-LOX pathway, so increasing the production of gastrotoxic leukotrienes.
Inhibition of 5-LOX may therefore offer a new approach to reducing the GI
toxicity associated with NSAID use, while retaining the analgesic and anti-inflammatory properties of NSAIDs and COX-2 specific inhibitors.
In comparison with naproxen 500mg twice daily, administration of licofelone (200mg to 400mg twice daily) was associated with a substantially reduced incidence and severity of duodenal erosion and ulceration. Whereas 20% of naproxen-treated subjects developed ulcers, none occurred in licofelone or placebo recipients. These findings appear consistent with the safety and tolerability data reported in earlier clinical trials in OA patients.
Interestingly, 5-LOX has been implicated in the deterioration of joints in OA. Inhibition of 5-LOX may therefore help protect arthroidal cartilage and slow disease progression. In preclinical studies licofelone was found to reduce the expression of matrix metalloproteinases, cathepsin K and IL-1beta, and help prevent chondrocyte death, indicative of potential disease-modifying effects in OA.
Licofelone shows clinical efficacy
Clinical studies that have compared licofelone with conventional NSAIDs and COX-2 specific inhibitors suggest that it is at least as effective as these agents in providing symptomatic relief from OA but generally better tolerated.
In a 12-week study, in which licofelone 200mg was compared with naproxen 500mg in 148 OA patients, 69.4% of licofelone-treated patients responded to treatment compared with 68.4% in the naproxen arm. Response was defined as a 30% improvement from baseline on the Western Ontario McMaster Universities Osteoarthritis (WOMAC) Index.
While there was no difference in efficacy between treatments, twice as many naproxen-treated patients experienced adverse GI effects compared with those on licofelone. A longer, 52-week, comparative study of licofelone (100mg and 200mg twice daily) and naproxen (500mg twice daily) in 704 OA patients again showed similar levels of efficacy between treatments but greater tolerability among licofelone recipients.
A comparative study of licofelone and celecoxib in 608 patients with symptomatic OA of the knee, in which patients were randomised to 200mg of either drug twice daily for four weeks, showed no difference in efficacy as measured on the WOMAC Index. However, fewer patients in the licofelone arm experienced adverse events compared with those receiving celecoxib.
Sumitomo to develop and market Licofelone in Japan
An agreement was reached in May 2004 between Sumitomo Pharmaceuticals and Merckle GmbH, in which Sumitomo secured the rights to develop and market licofelone in Japan. Because licofelone has a different mode of action to currently marketed NSAIDs, the companies hope it will be seen as an entirely new treatment option for OA patients. Almost 4 million people in Japan take NSAIDs for OA and related disorders.
Before licofelone reaches the Japanese market however, clinical trials have to be undertaken and regulatory approval secured.
Marketing commentary
Drugs for relief of pain and inflammation constitute a large segment of the CNS market, in which COX-2 specific inhibitors are a growing sector. Clinical trial data indicate that, in common with COX-2 specific inhibitors, licofelone also offers GI safety advantages over existing NSAIDs for OA patients, most of whom require long-term treatment with anti-inflammatory drugs.
If dual COX/LOX inhibitors such as licofelone can also protect arthrodial cartilage and slow disease progression, a property not conferred by treatment with conventional NSAIDs or COX-2 specific inhibitors, then that could give them a competitive edge in a fiercely contested market.