Under development by Pfizer, torcetrapib is a member of the cholesteryl ester transfer protein (CETP) inhibitor class of drugs that is designed to boost blood levels of high-density lipoprotein (HDL), often described as “good cholesterol”. The company was exploring the potential to combine torcetrapib with its top selling statin Lipitor (atorvastatin) as an improved treatment for dyslipidaemia.
The combination of torcetrapib and Lipitor (atorvastatin) was in pivotal phase III trials involving about 15,000 patients, which were expected to run for at least two years. However, Pfizer was forced to stop the trials early after an independent safety board discovered an imbalance in the incidence of cardiovascular events and death in the two treatment arms (combination therapy versus atorvastatin alone). Further clinical evaluation of torcetrapib has ceased bringing to an end the development of a drug that Pfizer had hoped to submit for regulatory approval in 2007.
Despite this significant setback, Pfizer still has other combination therapies in its development pipeline for treatment of cardiovascular disease (CVD). They include Caduet, a combination of Lipitor and the anti-hypertensive agent Norvasc, and the ACAT inhibitor avasimbe. These products, designed to expand Pfizer’s CVD franchise, are part of a new approach to regulating blood plasma levels that goes beyond statin control.
DYSLIPIDAEMIA AND THE BURDEN OF CARDIOVASCULAR DISEASE
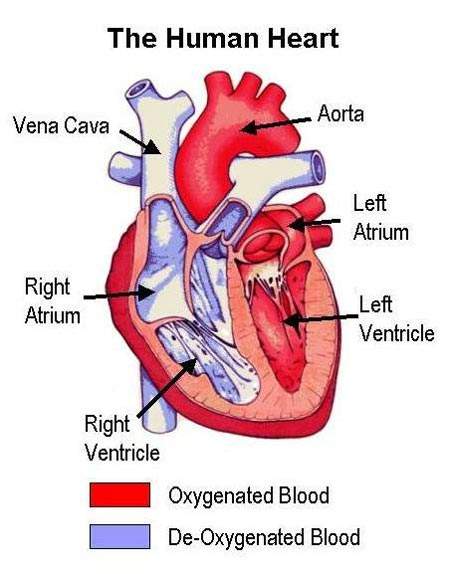
Coronary heart disease (CHD), the physical manifestation of atherosclerosis, is a major cause of death and disability. Of the 17 million deaths that occur from CVD in the world each year, CHD is a significant contributor.
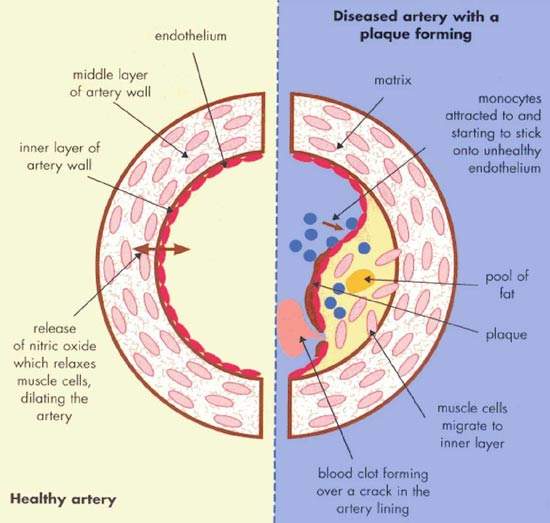
Atherosclerosis is a pathological process in which arteries supplying the heart become progressively narrowed by the build up of fatty material in the artery wall. Known as atherosclerotic plaques, they lead to narrowing or stenosis of the artery lumen so restricting blood flow to the heart. A lack of oxygen supply to the heart leads to adverse clinical consequences such as angina, heart attack (myocardial infarction) and sudden cardiac death.
An abnormality in blood lipid and lipoprotein levels, or dyslipidaemia, is known to predispose to CHD. Treatment is therefore aimed at correcting lipid abnormalities to reduce the risk of CHD developing in individuals without overt heart disease (primary prevention) as well as reducing the risk of adverse cardiac events occurring in those with CHD (secondary prevention). Initially the focus was on reducing plasma levels of atherogenic lipids and lipoproteins such as low-density lipoprotein cholesterol (LDL-C) and triglycerides. Attention is now turning to therapies which can boost the levels of cardioprotective lipoproteins, such as high-density lipoprotein (HDL).
CETP AS A THERAPEUTIC TARGET FOR DYSLIPIDAEMIA
CETP acts as a key intermediary in the distribution of cholesterol between high- and low-density lipoproteins (HDL, LDL). Pfizer’s torcetrapib was designed to inhibit CETP.
In theory, by blocking CETP it should be possible to improve the ratio of HDL-C to LDL-C, the so-called atherogenic index, and thereby slow the development and progression of atherosclerosis.
COMBINATION THERAPY FOR DYSLIPIDAEMIA CONTROL
Following their introduction into clinical practice over 20 years ago, the statins rapidly superseded older lipid-lowering drugs to become the treatment of choice for most patients with dyslipidaemia. Today they underpin the management of dyslipidaemia. A wealth of data shows they can have a significant impact on CHD-related morbidity and mortality.
Statins are potent reducers of atherogenic LDL-C but have a more modest effect on raising levels of cardioprotective HDL-C. Pfizer believed that a combination of Lipitor with torcetrapib could potentially raise levels of cardioprotective HDL by as much as 50%, while simultaneously lowering atherogenic LDL by 70–80%.
Early phase II data published in April 2004, showed that inhibiting CETP with torcetrapib 120mg/d produced significant increases in HDL-C, when given alone (46%) as well as in combination with Lipitor (61%), while also reducing LDL-C by more than that achieved with Lipitor alone. When the dosage of torcetrapib was increased to 240mg/d, HDL-C increased by over 100%. At this stage, combination therapy appeared safe and well tolerated.
Large-scale phase III trials evaluating the efficacy and safety of torcetrapib in combination with Lipitor followed. One of the first was a multicentre randomised trial that compared atorvastatin/ torcetrapib combination therapy with atorvastatin alone in preventing progression of coronary atherosclerosis as measured by intravascular ultrasound (IVUS) in patients with non-obstructive CHD (20-50% stenosis). Patients received atorvastatin first to lower plasma LDL-C levels before randomisation to combination therapy or atorvastatin alone.
In light of safety concerns, Pfizer has cancelled the atorvastatin/ torcetrapib combination therapy trials.
MARKETING COMMENTARY
Dominated by sales of statins, the market for drugs to treat dyslipidaemia is the largest and most dynamic sector of the CVD market. This is projected to continue, driven by large treatment gaps from under-diagnosis and under-treatment of dyslipidaemia as well as a rising prevalence of obesity and diabetes (risk factors for CHD). Combination therapies, many involving compounds containing a statin, are among the new treatment paradigms expected to emerge over the next ten years. These new therapies have the potential to achieve even greater efficacy than that achieved with today’s statins. As the market for statins matures with the introduction of generic versions, companies will be seeking ways to retain and build market share. A single pill containing a statin/non-statin combination may fulfil that role.