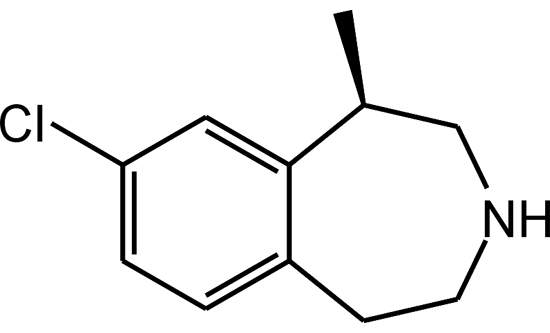
Lorcaserin hydrochloride (Lorcaserin) is an investigative oral treatment drug for obesity, and was discovered by Arena Pharmaceuticals (Arena), a clinical-stage biopharmaceutical company, which also holds the drug patent.
A selective agonist of 5-HT2C serotonin receptors, the drug was initially designated by Arena as APD356. The drug maker claims that lorcaserin hydrochloride selectively stimulates the 5-HT2C receptors located in the brain’s hypothalamus region, which assists in appetite suppression and metabolism activities. The drug has also been found to have about 100-fold selectivity in vitro for the 5-HT2C receptors compared with the 5-HT2B receptors. The selectivity in vitro is 15-fold in the 5-HT2C receptors when compared with that of the 5-HT2A receptors.
Lorcaserin is one of Arena’s four internally discovered drug candidates. In December 2009, Arena submitted a new drug application (NDA) for Lorcaserin to the Food and Drug Administration (FDA).
In February 2010, the NDA submission was accepted by the FDA. In the same month, Arena announced that it had received a Prescription Drug User Fee Act (PDUFA) date of 22 October 2010 for the drug.
On 25 October 2010, the FDA rejected Arena’s NDA for Lorcaserin. In a detailed letter sent to the company, the FDA instructed Arena to furnish additional information on the preclinical study conducted on rats, which revealed a correlation between the drug and the occurrence of cancer. The FDA also mentioned that the efficacy of the drug in non-diabetic overweight and obese subjects was marginal and asked the company to submit the final results of the recently concluded BLOOM-DM study.
Arena had expected to release the drug in 2010 at estimated initial prescriptions of ten million, which would have had $1bn revenue potential.
Phase III clinical trials
The Phase III clinical trials on Lorcaserin’s effectiveness were initiated in September 2006 and included three programmes. The first clinical trial programme was called Behavioural Modification and Lorcaserin for Overweight and Obesity Management (BLOOM). The second and third trials on safety and efficacy of the drug were initiated in December 2007. The trials were named Behavioural Modification and Lorcaserin Second Study for Obesity Management (BLOSSOM) and BLOOM in Diabetes Mellitus (BLOOM DM) respectively.
The BLOOM trials were conducted involving approximately 3,200 patients in about 100 locations in the US. The double-blind randomised placebo-controlled trial evaluated the efficacy of a 20mg daily dose (10mg in two doses daily) of Lorcaserin over a two-year treatment period, over the placebo. The trials were conducted in two scenarios; one on obese patients with a BMI of 30 to 45 with or without co-morbid conditions, and another on overweight patients with a BMI of 27 to less than 30 with at least one co-morbid condition.
On 6 June 2009 Arena announced positive results from the first stage of its Phase III trials. According to the results presented, Lorcaserin has demonstrated significant categorical and absolute weight loss, and improvements in multiple secondary endpoints related to cardiovascular risk, as compared to placebo.
The BLOSSOM trial was designed to evaluate efficacy of 10mg and 20mg daily doses of Arena’s drug over the placebo. Taking place in nearly 100 locations in the US, the trials were conducted over a one-year treatment period under the two scenarios, as in the BLOOM trials. A total of 4,008 patients were enrolled for the BLOSSOM trials.
The BLOOM-DM trial was also designed to be a similar evaluation over a one-year treatment period, but was conducted on obese and overweight patients with type 2 diabetes. The trials were performed at 60 sites in the US. Other indicators of glycaemic control were also studied in the BLOOM-DM trial, in addition to weight loss as the primary endpoint in testing efficacy.
Enrolment for BLOOM-DM was completed in 2009. About 600 patients were enrolled in the programme, which is a supplement to the NDA, while the Phase 3 trials enrolled about 7,800 patients in total.
Phase IIb trial results – high effectiveness of Lorcaserin
The Phase II clinical trials of the drug indicated favourable results on the efficacy of Lorcaserin on patients who were dosed up to 12 weeks. The test results, as Arena claimed, confirmed that Lorcaserin-dosed patients lost more weight than those who were administered the placebo.
In June 2006, the results of Phase IIb studies on Lorcaserin conducted by its principal investigator Steven Smith were presented in Washington, DC at the 66th Annual Scientific Sessions of the American Diabetes Association (ADA).
The test results concluded that patients who completed the 12-week treatment period with the drug at daily doses of 10mg, 15mg and 20mg (10mg administered twice everyday), respectively achieved a statistically significant (p<0.001) mean weight loss of 4lb, 5.7lb and 7.9lb.
The weight loss figures indicated a higher effectiveness of Lorcaserin compared with 0.7lb for the placebo class. Similar improvements in the Phase IIb results were found in BMI and waist circumference.
Designed as a randomised, double-blind, dose-ranging trial at about 40 locations in the US, the Phase IIb trial was conducted on 469 male and female obese patients whose BMI ranged from 29 to 46.
A study in the successfully completed Phase II trials on patients administered with 20mg of Lorcaserin daily concluded that the drug resulted in a mean weight loss of 7.9lb, while the placebo helped to lose just under 1lb of weight.
Safety and tolerance
The safety profile of Lorcaserin on adults was favourable, in conclusion of the Phase II clinical trials. The drug was found to have no side effects on heart valves or pulmonary artery vasculature, and was well tolerated at all doses. The last observation carried forward (LOCF) analysis for each year on frequently generated echocardiograms confirmed the non-association of Lorcaserin with valvular insufficiency, and has paved way for the company to meet the FDA requirements.
However, the drug caused minor side effects such as headaches, dizziness and nausea in human clinical trials. Headaches have been the most common side effect.
The first Phase III clinical trial programme results announced in June 2009 confirmed that the drug produced a comparably lower rate of depression-related events over the placebo during the first two years. Further, the discontinuation rates for adverse events for year one and two in the lorcaserin and placebo were similar with 7.1% versus 6.7% and 3.0% versus 3.0%, respectively, for the two groups.
Marketing commentary
According to the National Institutes of Health report in 2007, nearly 65% of the US adults are obese or overweight. A 2005 report by the International Diabetes Federation mentioned that medical and related costs of obesity are $123bn per year in the US.
As of March 2009, Meridia and Xenical were the only two approved chronic use drugs in the US to treat obesity apart from Alli, an over-the-counter low-dose version brand of Xenical. Increasing obesity and related problems have caught the attention of pharmaceutical companies such as Arena in the growing anti-obesity drugs market.
Arena’s primary focus, however, includes four major therapeutic areas: metabolic, central nervous system, cardiovascular and inflammatory diseases. The clinical-stage biopharmaceutical company is engaged in the discovery, development and commercialisation of small molecule drugs in these areas.