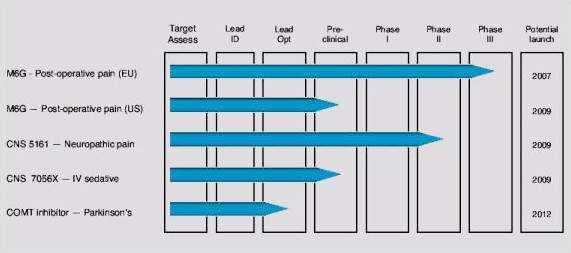
Morphine-6-Glucuronide (M6G) is a new morphine derivative under development by biopharmaceutical company PAION. Development of M6G was initiated by CeNeS Pharmaceuticals, which PAION acquired in June 2008. CeNeS was a UK company specialising in the development of drugs that act on the central nervous system (CNS).
M6G is the company’s lead compound and indicated for the treatment of moderate to severe post-operative pain. The US Food and Drug Administration approved the M6G investigational new drug application submitted by CeNeS in April 2007.
Successful completion of a pivotal pan-European trial showed that while M6G provides comparable analgesia to morphine, the incidence of nausea and vomiting is significantly reduced.
The drug could not demonstrate significant results regarding the incidence and severity of nausea. However, a meta-analysis of Phase II and Phase III clinical data conducted by PAION post-acquisition has confirmed the analgesic effect and wider therapeutic margin of M6G over morphine. PAION is now in search of a development partner for the drug.
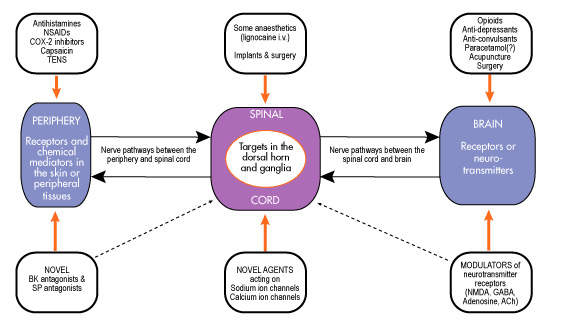
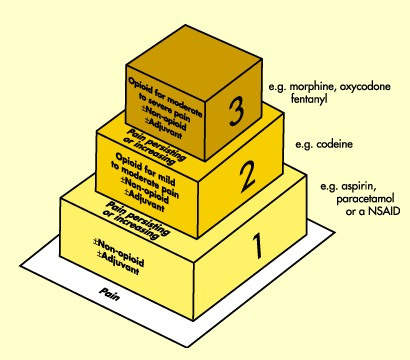
Opioids underpin management of severe post-operative pain
Discovered at the turn of the 19th century, opioids such as morphine and morphine derivatives are arguably the oldest class of pain-relieving drugs. Today, they remain the “gold standard” for the treatment of severe pain, including post-operative pain and chronic pain associated with advanced disease.
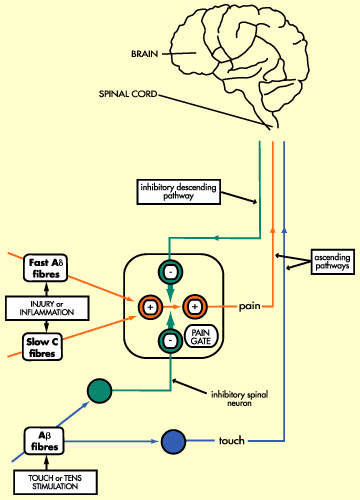
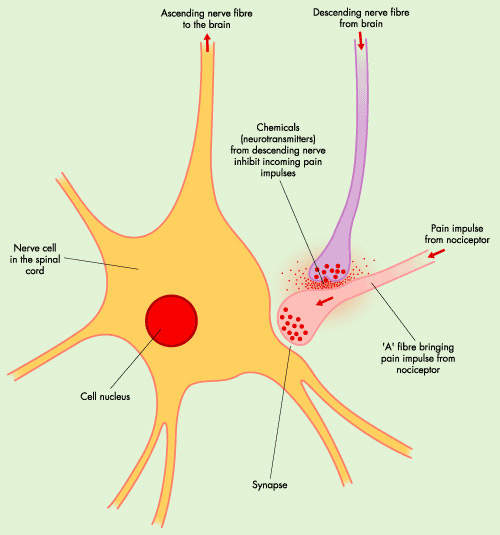
Opioids, originally derived from a species of poppy, act on receptors within the brain to mimic the actions of naturally occurring opioids (endogenous opioids). Morphine is a highly potent opioid drug and one of the most effective analgesics currently available. However, it is associated with a number of unwanted side effects and is often poorly tolerated by patients. Adverse effects associated with morphine use include nausea and vomiting, sedation and respiratory distress.
For the many millions of patients who undergo surgery in Europe and the US each year, there is a need for opioid derivatives with an improved safety and tolerability profile. PAION’s M6G has been developed to meet that need.
M6G reduces morphine side-effect burden
Clinical studies in which M6G has been compared with conventional morphine preparations in the post-operative setting show that it possesses similar analgesic efficacy, but with a significantly improved side-effect profile.
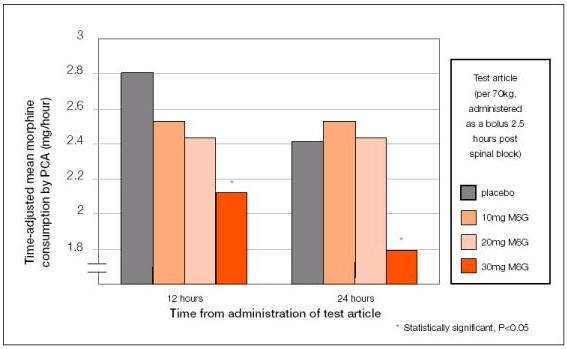
Early evidence for a reduction in side-effect burden following treatment with M6G emerged in Phase II studies, which showed a lower incidence of adverse events compared with morphine. In patients admitted for day-case gynaecological surgery, for example, the incidence of nausea and vomiting was reduced by more than 50% when compared with morphine for control of post-operative pain.
Larger Phase III trials have since confirmed these encouraging early results. In a randomised, double-blind trial in which M6G was compared with morphine in patients undergoing major orthopaedic surgery, there was a marked reduction in side-effect burden in the M6G treatment arm. In addition to a 50% reduction in the incidence of nausea and vomiting among patients receiving M6G, incidence of respiratory depression and sedation was also markedly lower.
These findings have now been underpinned by data from the Phase III European registration trial in 500 patients undergoing open abdominal surgery (hysterectomy, major GI, bowel and urological).
Powered to test for non-inferiority between M6G and morphine treatments with respect to analgesic efficacy and for superiority with respect to side-effect profiles, the results showed that M6G was equivalent to morphine in providing pain relief but associated with significantly lower rates of
post-operative nausea and vomiting. Patients in the M6G treatment arm experienced a 28% reduction in the severity of post-operative nausea and vomiting (p=0.018) and a 32% reduction in dry retching (p=0.044).
Patients enrolled in the Phase III trial received an initial bolus dose of M6G or morphine followed by patient-controlled analgesia (PCA) with either M6G or morphine.
PAION also targets neuropathic pain
In addition to M6G, PAION is developing CNS 5161 for the treatment of neuropathic pain. Neuropathic pain is chronic pain that arises from damage to sensory nerves and often doesn’t respond to treatment with opioid or non-steroidal anti-inflammatory drugs (NSAIDs). It can include:
- Pain arising from trapped or compressed nerves
- Drug-induced nerve damage
- Diabetic neuropathy
- Post-herpetic pain
- Phantom limb syndrome following limb amputation
- Peripheral neuropathy
- Fibromyalgia
CNS 5161 is a selective non-competitive N-methyl D-aspartate (NMDA) receptor antagonist that binds to a site within the ion-channel associated with the NMDA receptor. Initial, small-scale clinical trials have shown evidence of efficacy and good toleration in patients with chronic intractable neuropathic pain.
Marketing commentary
Drugs for analgesia are an important segment of the global CNS market. Opioids are an established class of pain-relieving drugs and the mainstay of management of severe pain, including severe post-operative pain.
With evidence to suggest M6G has comparable efficacy to conventional morphine preparations but an improved side-effect profile, it is well placed to succeed. Securing a licensing agreement with a major pharmaceutical company to market M6G will be an important determinant of its success.