Mavenclad™ (cladribine tablets) is a selective immune reconstitution therapy indicated for the treatment of active relapsing multiple sclerosis.
Discovered and developed by Merck Group, it is one of the first approved oral short-course treatments.
Merck received positive opinion for the approval of Mavenclad from the Committee for Medicinal Products for Human Use (CHMP) of the European Medical Association (EMA) in June 2017.
The European Commission (EC) granted marketing authorisation (MA) for Mavenclad in August 2017 and the drug was launched in all 28 European countries, including Norway, Liechtenstein and Iceland.
Merck also received Canadian approval for the drug from Health Canada, while the Therapeutic Goods Administration (TGA) authorities approved the drug in Australia in December 2017.
The company plans to file marketing approval for the drug in the US in 2018.
Multiple Sclerosis causes and symptoms
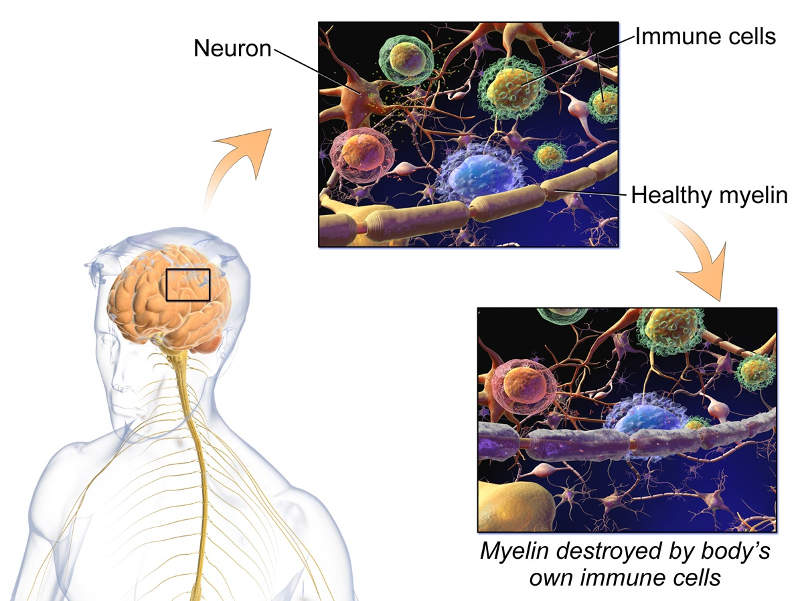
Multiple sclerosis (MS) is a neurological disorder that affects the brain or spinal cord of the central nervous system (CNS).
The disease is caused by the damage of the myelin sheath, which surrounds and protects the nerves cells. The disease is more commonly found in women aged between 20 and 40 years.
Relapsing-remitting MS is the most common form of the disease and accounts for approximately 85% of cases. The disease currently has no cure, with existing treatments providing only symptomatic relief.
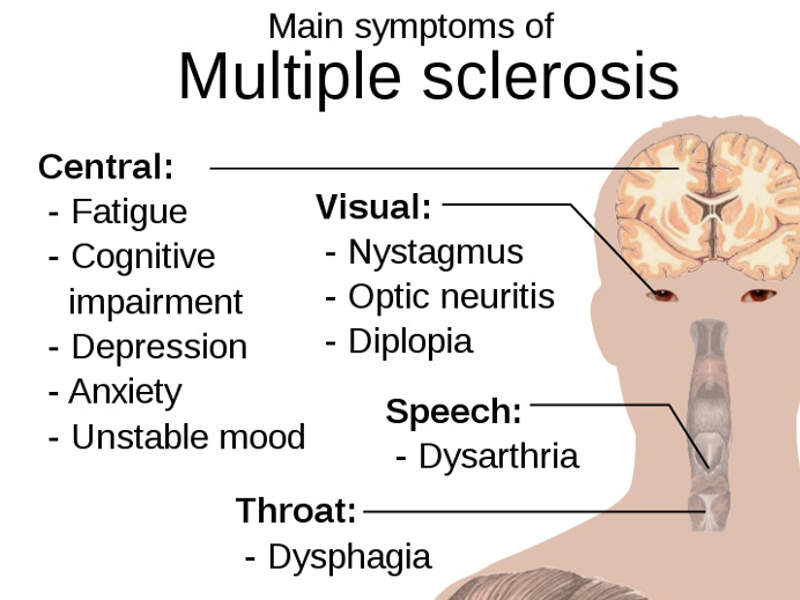
The most common symptoms associated with the disease include fatigue, blurred vision, numbness in different parts of the body, muscle stiffness and spasms, as well as issues with cognition.
According to the European MS Platform, more than 700,000 people across Europe and 23,000 in Australia are currently affected by the disease.
Other medications approved for the treatment of MS are Tecfidera developed by Biogen Idec and Glatopa manufactured by Sandoz and Momenta.
Mavenclad mechanism of action
Mavenclad contains a selective immune reconstitution therapy that works by targeting B & T lymphocytes. It helps in the rapid reduction of natural killer cells with minimal impact on neutrophils, platelets and monocytes.
The drug is available in 10mg dose tablets for oral administration.
Clinical trials on Mavenclad
The MA granted by the EC for Mavenclad was based on results obtained from a clinical trial programme involving more than 2,700 patients and ten years of observation.
The clinical trial programme included data obtained from Phase III clinical trials Cladribine Tablets Treating MS Orally (CLARITY) and its extension, as well as Oral Cladribine in Early MS (ORACLE MS) and Phase II trial Oral Cladribine Added ON To Interferon beta-1a in Patients With Active Relapsing Disease (ONWARD).
Long-term follow-up data from the eight-year prospective registry was also used to review the drug.
The clinical studies evaluated the efficacy and safety of Mavenclad in MS patients. Results from the CLARITY trial demonstrated that patients administered with Mavenclad experienced a 67% reduction of the annualised relapse rate and an 82% reduction in the risk of six-month confirmed expanded disability status scale (EDSS) progression compared to placebo.
Results from the Phase III CLARITY extension study showed that no further Mavenclad treatment was required in years three and four.
The most adverse reactions reported in patients treated with Mavenclad in clinical studies were lymphopenia and herpes zoster.
Marketing commentary on Merck
Headquartered in Darmstadt, Germany, Merck Group is involved in the development of healthcare, life science and performance materials.
The company develops biopharmaceutical therapies for the treatment of cancer and MS. It operates as EMD Serono, MilliporeSigma and EMD Performance Materials in Canada and the US.