Monovisc (sodium hyaluronate), indicated for the treatment of Osteoarthritis (OA) of the knee, is the first US Food and Drug Administration (FDA) approved single injection made with hyaluronate from a non-animal source. The drug was discovered and developed by Anika Therapeutics.
Monovisc was approved by the US FDA in February 2014 for treatment of pain and to improve joint mobility in patients suffering from osteoarthritis of the knee.
DePuy Mitek, a leading orthopedic sports medicine company entered into licence agreement with Anika Therapeutics for the commercial sale of Monovisc in the US. Under the agreement DePuy Mitek will pay $5m to Anika Therapeutics as milestone payment upon first commercial sale of Monovisc. DePuy Synthes Mitek Sports Medicine commercially launched Monovisc in the US and made the first sale in April 2014.
Monovisc is also sold in Canada, the UK, and a number of countries across the Middle East, Europe and Asia.
Osteoarthritis causes and symptoms
Otezla (apremilast) is an inflammatory drug indicated for the treatment of adult patients with active psoriatic arthritis (PsA).
Osteoarthritis is a disease of joints of the knee, which is caused by aging joints, injury and obesity. The disease occurs due to the breakdown of cartilage, which is part of the joint that cushions the knee bone ends. The symptoms of the disease include pain in the affected joints, and stiffness. The disease commonly affects middle aged and older people.
More than five percent of the people in the world are estimated to be affected by osteoarthritis of the knee. The disease also affects about 10 million people in the US.
Monovisc’s mechanism of action
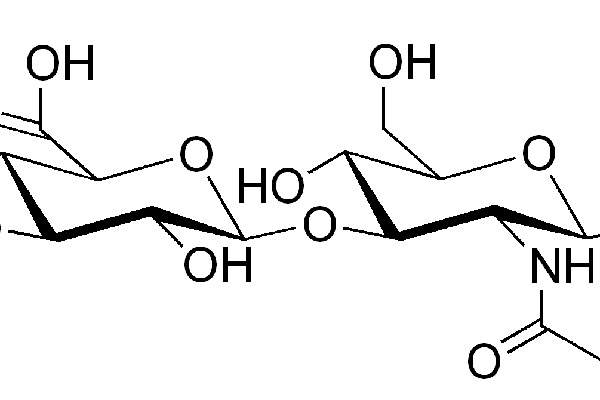
Monovisc contains sterile, non-pyrogenic, viscoelastic solution of sodium hyaluronan (NaHA). The drug is made from bacterial cells and includes combination of a proprietary cross-linker. The drug replaces natural hyaluronic acid in the body and provides significant pain relief from the symptoms of OA.
The drug is available in a disposable glass syringe of 4.0ml dose that can be administered through intra-articular injection.
Clinical trials on Monovisc
FDA approval of Monovisc was based a pivotal phase III clinical trial. The randomised, controlled, double-blind and multi centre clinical study enrolled 369 patients with OA of the knee in 31 centres from the US and Canada. The objective of the study was to evaluate the safety and effectiveness of Monovisc for the treatment of joint pain.
Patients were randomised to receive either Monovisc or control (saline injection). They were evaluated for improvement in pain based on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) after 26 weeks of the study.
The primary effectiveness analysis showed that the patients treated with Monovisc achieved a greater improvement from baseline in WOMAC pain score versus control after 12 weeks. The safety analysis of the study showed that Monovisc had an extremely low rate of adverse events. No serious adverse events were reported in Monovisc group during the clinical study.
Marketing commentary
Anika Therapeutics, one of the leading pharmaceutical companies in the US, is headquartered at Bedford in Massachusetts. The company is engaged in the production, marketing and commercialisation of therapeutic products for tissue protection, healing and repair. The company’s products are based on hyaluronic acid (HA) technology. The products include orthopaedic or joint health solutions, aesthetic dermal fillers, as well as surgical aids in the anti-adhesion and ophthalmic fields.
Other medications currently being investigated for the same indication include Licofelone developed by Merckle and its EuroAlliance partners – Alfa Wassermann and Lacer.