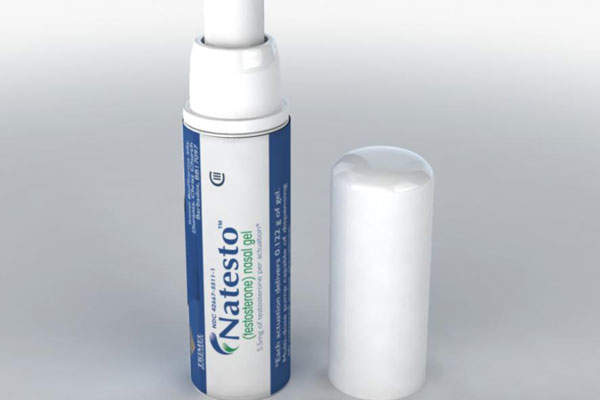
Natesto (testosterone, formerly known as CompleoTRT) is the only FDA-approved nasal gel for treatment of adult males with conditions associated with deficiency or absence of endogenous testosterone. It was developed by Trimel Pharmaceuticals Corporation.
Trimel Pharmaceuticals received approval for Natesto from the US Food and Drug Administration (FDA) for treating adult males for conditions associated with a deficiency or absence of endogenous testosterone in May 2014.
Low testosterone or hypogonadism
A testosterone replacement therapy for hypogonadism in adult men who are associated with a deficiency or absence of the male hormone.
Low testosterone level in males is a condition also known as hypogonadism. It occurs when male testicles or pituitary gland is affected by disease or damage, which results in stopping hormone and testosterone secretion. Erectile dysfunction, decreased sexual desire, depressed mood, fatigue, osteoporosis and regression of secondary sexual characteristics are the symptoms associated with low testosterone levels.
It is estimated that around 13 million people across the US are affected by this problem.
Natesto’s mechanism of action
Natesto contains testosterone, a steroid hormone, which can be used as androgen replacement therapy for treatment of Primary hypogonadism and hypogonadotropic hypogonadism. Testosterone and dihydrotestosterone (DHT) are necessary for the normal growth and development of the male sex organs.
The drug is supplied as a gel that can be self-administered into the nostrils through a metred-dose pump applicator.
Clinical trials on Natesto
Trimel Pharmaceuticals evaluated the safety and efficacy of Natesto in a phase III clinical trial, which was a multicentre and open-label clinical study conducted for 90 days. The study enrolled 306 hypogonadal male patients across 39 clinical research centres in the US.
Patients enrolled for the study included Caucasian, African-American and Asian races with an average age of 54, and ages ranging from 28 to 80 years. Patients were administered with Natesto intranasally. In the study period, 78 patients were treated with 33mg of testosterone daily, with 73 patients included in the statistical evaluation of efficacy on day 90. The evaluation was based on the intent-to-treat (ITT) population with last observation carried forward (LOCF).
Study results showed that around 90% of patients administered with Natesto had an average serum total testosterone concentration (Cavg) within the normal range between 300ng/dL to 1,050ng/dL on day 90, while 10% of patients had Cavg below the normal range (lower than 300ng/dL). It was also observed in the study that no patient had exceeded Cavg value of 1,050ng/dL.
The most common adverse reactions found in the patients administered with Natesto during the clinical study included an increase in prostate specific antigen (PSA), headache, rhinorrhea, and epistaxis. The adverse reactions also included nasal discomfort, nasopharyngitis, bronchitis, upper respiratory tract infection, sinusitis and nasal scab.
Marketing commentary
Headquartered at Mississauga, Canada, Trimel Pharmaceuticals is a specialty pharmaceutical company engaged in developing medications for male hypogonadism, female sexual dysfunction and a range of other respiratory disorders.
Other medications available in the market for same indication include Aveed (testosterone undecanoate) developed by Endo Pharmaceuticals and Bayer Pharma.