NicVAX is an investigational vaccine developed for use as an aid to treat smoking addiction and prevent smoking relapses. The vaccine is being developed by Nabi Biopharmaceuticals.
Nabi has received several patents for the vaccine including a recent patent in August 2010 for treating and preventing nicotine addiction.
In February 2006, NicVAX was granted the US Food and Drug Administration’s (FDA) fast track status for smoking cessation products.
In September 2005, Nabi was awarded a $4.1m grant by the US National Institute on Drug Abuse (NIDA) for the NicVAX development programme. A $10m grant was received from NIDA in September 2009 for further development of the vaccine.
Nabi has also received a grant of $244,479 for the development of the drug from Internal Revenue Service.
Nicotine addiction and smoking relapse
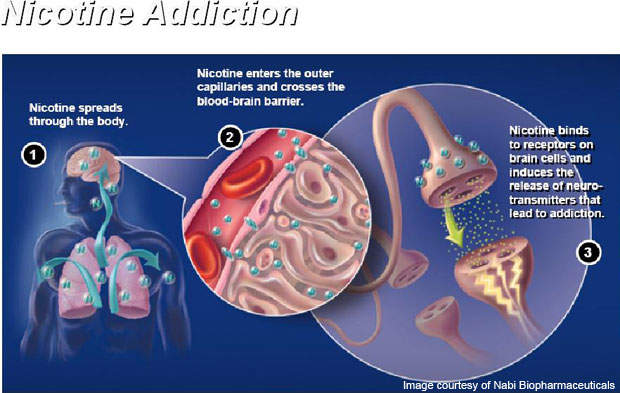
Nicotine is the addictive drug in cigarettes, which enters into the bloodstream and reaches the brain.
When a person smokes a cigarette the body responds to chemical nicotine present in the smoke, which causes an increase in heart rate, blood pressure and flow of blood from the heart.
In addition to multiple harmful and carcinogenic agents, cigarette smoke includes carbon monoxide that adversely affects the oxygen-carrying capacity of the blood.
Many people experience smoking relapse at some point when they attempt to quit smoking.
Smoking is a healthcare problem that looms large over the world. The WHO has estimated that there were more than 1.3 billion smokers worldwide and more than five million tobacco related deaths are occurring every year.
Tobacco is the cause of more than 500,000 deaths in the US, and more than 700,000 deaths in China.
Smoking is responsible for 30% of all cancer deaths. Lung cancer is the leading cause, which kills about 157,000 people in the US annually. Smoking is the main risk factor, causing more than 80% of the lung cancer deaths.
NicVAX
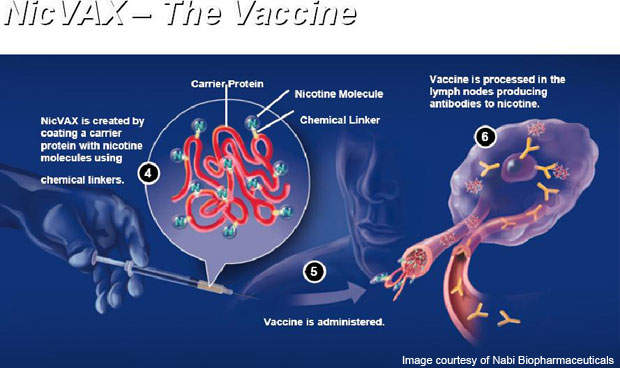
NicVAX is a nicotine conjugate vaccine, which is intended to reduce or eliminate physical nicotine addiction. The vaccine contains nicotine-specific anti-nicotine bodies, which can assist this process. It can also be used as a preventative measure to protect the body against addiction. The drug consists of hapten 3′-aminomethylnicotine mixed with recombinant Pseudomonas aeruginosa exoprotein A.
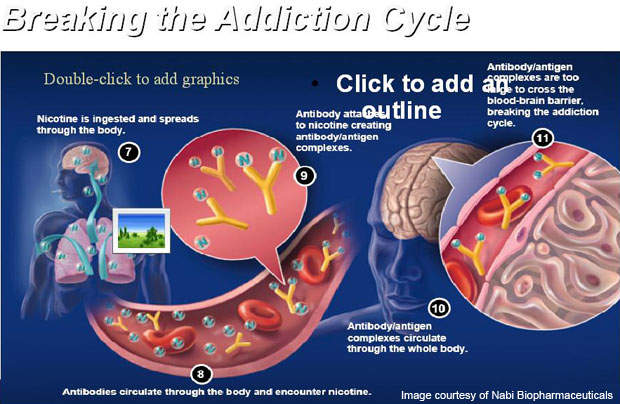
The vaccine is administered through six injections over a period of six months. It works by stimulating the immune system of the body to produce specific anti-bodies. The antibodies contained in the vaccine attach themselves to nicotine molecules to make the molecules in the bloodstream too big to cross the blood-brain barrier.
Clinical trials
Nabi started a Phase II clinical trial on NicVAX in May 2006 and the study concluded in September 2007 with final data collection and primary outcome measure. The study was conducted on 313 smokers who were in good general health, smoked at least fifteen cigarettes a day, with carbon monoxide levels of more than 10ppm, and were ready to give up smoking.
The primary outcome measure studied was continuous smoking abstinence. The secondary outcome measure studied point prevalence abstinence in a time frame of 24 months.
Nabi is currently conducting a phase IIB clinical study on NicVAX. The multicenter, randomised, double-blinded, parallel-arm study has enrolled 600 subjects. It is being carried out in the Netherlands and will evaluate NicVAX in combination with varenicline (Chantix). The study is expected to be completed by second half of 2012.
In late 2008, Nabi reached an agreement with the FDA on a special protocol assessment for Phase III NicVAX clinical trials. The first Phase III clinical trials on the drug were started in November 2009 across 22 centres in the US. It was a double-blinded, placebo-controlled trial. .
The study enrolled 1,000 current smokers who were in good general health, smoke at least ten cigarettes a day and were willing to give it up.
The primary outcome measure of the study was to evaluate the drug as an aid to smoking cessation and long-term abstinence.
The secondary outcome measures included evaluating abstinence rates at multiple intervals. The study also assessed the safety and immunogenicity of the drug, and appraised the smoking satisfaction, cigarette consumption, nicotine dependence and withdrawal symptoms. Smoking abstinence was measured using various methods, including as exhaled carbon monoxide and self-reported cigarette consumption.
Results of the study were announced in July 2011. The results demonstrated that NicVAX failed to meet the primary endpoint of the study.
An identical second Phase III trial began in March 2010. It was a double-blinded, placebo-controlled trial which enrolled about 1,000 subjects. Enrolment for this trial was completed in November 2010. The primary endpoint of the trial was evaluating the abstinence rate for 16 weeks.
Results of the study, announced in November 2011, showed that the drug failed to meet the primary endpoint.
Marketing commentary
In November 2009, Nabi and GlaxoSmithKline Biologicals (GSK) announced a worldwide option and licensing agreement for NicVAX and a licence to develop next generation vaccines using Nabi’s intellectual property. The company received $40m from GSK at the transaction close. In addition, Nabi is eligible to receive up to $460m option fees and milestone payments, as well as royalties on global sales of NicVAX and next-generation nicotine vaccines developed by GSK.
The smoking cessation market worldwide has been growing at a compounding annual growth rate of 11%, reaching an estimated market size of $3.8bn by 2018.
The CDC researchers estimated that 70% of smokers want to give up the habit but less than 5% of them were able to quit smoking. If approved, NicVAX vaccine would help Nabi tap the huge smoking cessation market.