Iloperidone is a dual-acting dopamine and serotonin receptor antagonist, developed originally by Titan Pharmaceuticals as a new treatment for schizophrenia. For several years Novartis Pharma held the worldwide development and marketing rights to iloperidone, but the company relinquished these rights in 2003.
Subsequently, the rights were taken up by Vanda Pharmaceuticals. Under the license agreement from Novartis, Vanda Pharmaceuticals undertook to pursue the iloperidone Phase III development programme and complete product registration.
In October 2007, Vanda Pharmaceuticals submitted a New Drug Application (NDA) to the US Food and Drug Administration (FDA) seeking approval for iloperidone for the treatment of schizophrenia. The NDA included data from 35 clinical trials involving more than 3,000 schizophrenia patients treated with iloperidone.
The FDA approved iloperidone in May 2009 for treatment of schizophrenia in elderly people. Iloperidone has been launched on the market under the brand name Fanapt. Titan Pharmaceuticals will obtain 8% royalty on worldwide annual net sales of up to $200m and 10% on net sales above $200m.
Iloperidone demonstrates efficacy in Phase III trials
Data from the Phase III trials indicate that consistent with its receptor binding profile, iloperidone is an effective treatment for schizophrenia.
In a randomised, double-blind, placebo-controlled trial involving 604 schizophrenia patients, treatment with iloperidone led to a significant improvement on all efficacy measures when compared with placebo. These included PANSS total (p=0.006), PANSS positive symptoms only (p=0.0009), PANSS negative symptoms only (p=0.027), and the Brief
Psychiatric Rating Scale (p=0.0128).
The efficacy of iloperidone was also assessed in relation to specific patient genetic profiling. The company had previously identified a genetic polymorphism, hypothesised to be associated with the pathogenesis of schizophrenia, in about 70% of patients and which appeared to correlate with iloperidone response. In this patient sub-set, the magnitude of response to treatment with iloperidone was greater than that for the total study population.
Cardiac safety assessed in Phase III trials
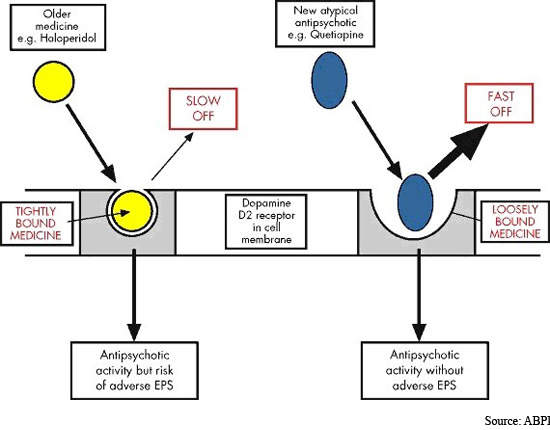
Safety and tolerability has been a major issue since antipsychotic drugs were first introduced into clinical practice over 50 years ago. Although atypical antipsychotics offer improved safety and tolerability over conventional typical antipsychotics, especially with respect to extrapyramidal symptoms (EPS), drug safety remains an issue.
Significant weight gain leading to metabolic disturbances (diabetes, heart disease), sexual dysfunction, hyperprolactinaemia and cardiotoxicity are unwelcome side effects associated with some currently used atypical drugs. Poor tolerability leads to poor treatment compliance and risk of relapse.
Cardiac safety has been a concern with iloperidone, particularly in relation to QT interval. Prolongation of the QTC interval, the length of time it takes the heart's ventricles to electrically discharge and recharge, is a documented side effect of several typical and atypical antipsychotics.
Cardiac safety data from the Phase III iloperidone trials revealed no patients had QT intervals in excess of 500ms, a threshold of concern to the FDA. Furthermore, among the sub-set of patients who responded particularly well to iloperidone, QT prolongation was shorter in the majority of patients who were good iloperidone metabolisers.
The change in QTC interval from baseline was 11.4ms for the total study population, 10.4ms for good metabolisers and 15ms for poor metabolisers (p=0.008, good vs. poor). In addition, iloperidone showed low potential for weight gain and induction of diabetes, low EPS including akathisia, and a low incidence of sleepiness and effects on cognition.
The burden of schizophrenia
Schizophrenia is a severe and debilitating mental illness that affects around 1% of the general population.
Worldwide, around 45 million people are afflicted with schizophrenia. This chronic and severe mental illness imposes a heavy burden on patients, their families and society.
Effective drug therapy can do much to ameliorate the symptoms of schizophrenia and reduce the need for inpatient care.
Residential care constitutes the single biggest component of the total cost of treating schizophrenia, which in the UK alone approaches £3bn a year.
By comparison, drug therapy is estimated to account for less than 10% of total costs.
Marketing commentary
Antipsychotic drugs are the cornerstone of the management of schizophrenia and are designed to address the core psychotic symptoms of schizophrenia. Despite a plethora of drugs, there are still significant unmet clinical needs in the management of schizophrenia.
Iloperidone is expected to expand the range of atypical antipsychotic drugs available to psychiatrists to treat this disabling condition.