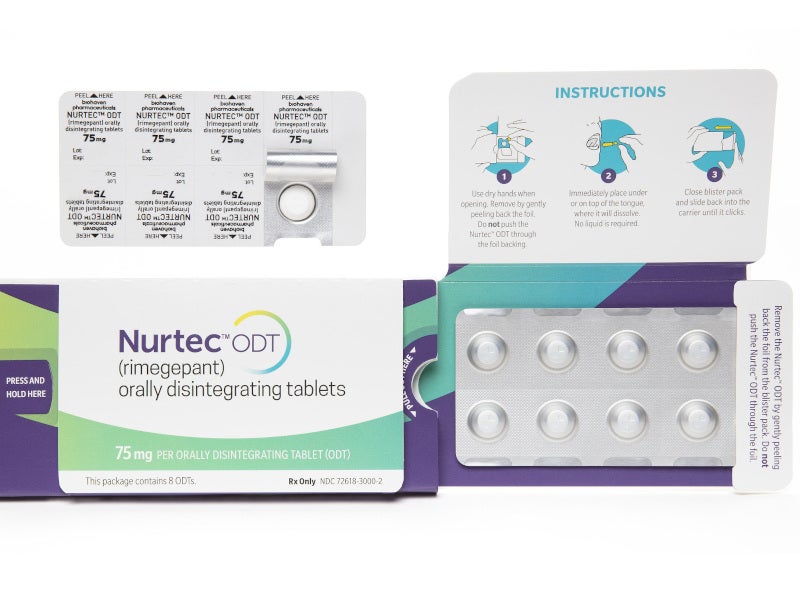
NURTEC® ODT (orally disintegrating tablet) (rimegepant) is the first oral calcitonin gene-related peptide (CGRP) receptor antagonist indicated for acute treatment of migraine with or without aura and preventive treatment of episodic migraine in adults.
NURTEC ODT, formerly known as BHV-300, was developed by Biohaven Pharmaceutical, a pharmaceutical company based in the US. Pfizer, a pharmaceutical company also based in the US, acquired the company in October 2022, leading to the addition of the breakthrough CGRP [calcitonin gene-related peptide] portfolio, including NURTEC ODT, into its portfolio.
NURTEC is available as a fast-acting ODT with a recommended dose of 75mg once daily. The drug is available in eight-tablet packs.
Regulatory approvals for NURTEC
Biohaven submitted two new drug applications to the US Food and Drug Administration (FDA) in June 2019.
The FDA approved NURTEC for the treatment of migraine with or without aura in adults, in February 2020 and for the preventive treatment of episodic migraine in May 2021.
NURTEC was approved in Europe under the trade name VYDURA® for both acute treatment of migraine and prevention of episodic migraine in adults in April 2022.
The Therapeutic Goods Administration of Australia approved rimegepant in July 2023 for acute migraine treatment and prophylactic treatment for episodic migraine in adults with at least four monthly migraine attacks.
Migraine causes and symptoms
Migraine is a neurological disorder characterised by moderate to severe throbbing pain typically on one side of the head. It can persist for hours or even days without appropriate treatment. The precise aetiology of migraines remains elusive, but they are believed to stem from transient alterations in the brain’s chemicals, blood vessels and nerves.
Accompanying symptoms often include nausea, vomiting and heightened sensitivity to sound and light. The disorder manifests in forms including migraine with aura, migraine without aura and silent migraine, each presenting its own set of challenges.
Rimegepant is a reversible inhibitor of the CGRP receptor, which reduces the effects of the CGRP neuropeptide. It functions by attenuating pain signalling, diminishing neurogenic inflammation and reducing arterial dilation, all without inducing active vasoconstriction.
By targeting CGRP receptors, which are integral to migraine pathophysiology, NURTEC® ODT effectively addresses the underlying cause of migraines with a single dose.
Clinical trials on NURTEC
The FDA approval of NURTEC was based on the positive results of Phase III randomised, multi-centre, double-blind and placebo-controlled study (study 1).
The study recruited 1,351 migraine patients who were randomised to receive either 75mg of NURTEC ODT or a placebo. The freedom from pain and the most bothersome symptom (MBS) were the co-primary endpoints of the study.
At two hours after a single dose of NURTEC ODT, 21.2% of patients treated with NURTEC® ODT achieved migraine pain freedom versus 10.9% on placebo. About 35.1% of patients treated with NURTEC ODT achieved freedom from MBS versus 26.8% on placebo.
NURTEC ODT, when compared to placebo, also showed relief from pain (36.8% versus 31.2%) and freedom from functional disability (22.3% versus 15.8%) within 60 minutes after treatment.
About 42.2% of patients on NURTEC ODT had sustained pain relief compared to 25.2% on placebo for up to two days.
The most common adverse reactions were severe rash, nausea, dyspnoea, hypersensitivity and abdominal pain/dyspepsia.
For the preventive treatment of episodic migraine in adults, rimegepant 75mg was evaluated in a multi-centre, double-blind, randomised, placebo-controlled study. The study enrolled 747 patients who were administered either rimegepant 75mg or placebo every other day. Efficacy was evaluated in 695 patients. The primary endpoint of the study was a change from baseline in the mean number of monthly migraine days (MMDs) during weeks nine through 12.
Patients who took rimegepant every other day achieved a 4.3-day reduction from baseline in mean MMDs versus a 3.5-day reduction in those taking placebo.
The drug is currently also being evaluated in different clinical trials for the treatment of other conditions.