Nymalize (nimodipine) is an oral liquid form capsule indicated to improve neurological outcomes in patients suffering from subarachnoid haemorrhage (SAH). It is developed by Arbor Pharmaceuticals, a specialty pharmaceutical company based in Atlanta, US.
Arbor filed the first new drug application (NDA) for Nymalize in December 2011. The US Food and Drug Administration (FDA) granted orphan designation to Nymalize and also provided seven years of market exclusivity to Abor for the drug.
In May 2013, Arbor received FDA approval for Nymalize for the improvement of neurological outcomes in adult patients with subarachnoid haemorrhage (SAH).
Arbor Pharmaceuticals currently holds the commercial rights of Nymalize in the US. The company plans to launch the drug in the US market in 2013.
Subarachnoid haemorrhage
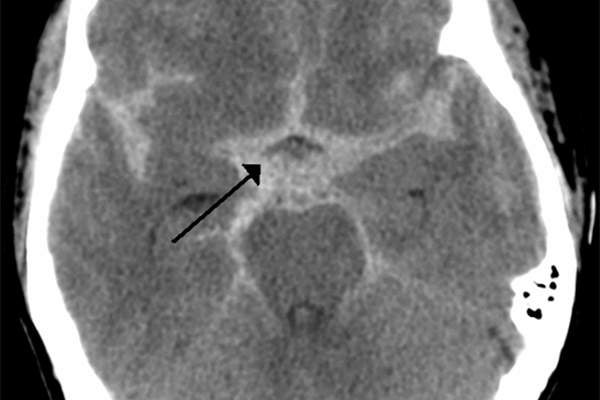
Subarachnoid haemorrhage is a type of fatal bleeding that occurs in the subarachnoid space, located between the brain and the thin tissues that cover it. The disease may be caused due to bleeding from an arteriovenous malformation or head injury, bleeding disorder, bleeding from a cerebral aneurysm or from the use of blood thinners. The symptoms of the disease include severe headache near the back of the head.
Subarachnoid haemorrhage is a rare disease that affects about 20 out of 100,000 people aged above 30 years in the US. The disease is found to be more common among women than in men.
Nymalize’s mechanism of action
The precise mechanism of action of the drug is not known but it is believed to work by blocking the dihydropyridine calcium channel. The drug stops the calcium ion transfer into muscle cells and blocks the contractions of vascular smooth muscle. The drug also reduces the incidence and severity of ischemic deficits in patients affected with SAH from ruptured intracranial berry aneurysms.
The drug is administered as an oral liquid form capsule in 20ml (60mg) dosage. It can also be administered through a nasogastric or gastric tube for a period of 21 days with a gap every four hours. The oral version of the drug is designed to reduce the prevalence of accidental intravenous (IV) injection of the drug, which can be fatal.
Clinical trials of Nymalize
The FDA approval for Nymalize was based on the outcomes from four randomised, double-blind, placebo-controlled Phase III clinical trials.
The first and second Phase III clinical trials were similarly designed. The studies enrolled unimpaired SAH patients and randomised them with Nymalize or placebo. The deficits due to spasm or other causes were graded.
The results of both studies showed that patients treated with Nymalize demonstrated significantly less severe deficits due to spasm when compared to the patients treated with placebo. In the second Phase III study the spasm-related deficits of all severities were fewer and no effect was found on deficits not related to spasm.
The third Phase III study enrolled 554 SAH patients with all grades of severity. The patients were administered with either Nymalize 60mg dose or placebo for every four hours.
The results of the study demonstrated that there was a significant reduction in the overall rate of brain infarction and severely disabling neurological outcome at three months.
The fourth Phase III clinical trial enrolled SAH patients who were much sicker, with Hunt and Hess grades. The patients were administered with Nymalize 90mg dose for every four hours.
The study results demonstrated that Nymalize-administered patients showed a significant reduction in spasm-related deficits. The rate of good recovery on the outcome scale was found to be 25.3% in Nymalize-administered patients, where as it was 10.9% in the placebo arm.
The adverse effects associated with Nymalize-administered patients during the clinical studies included hypotension, headache, nausea and bradycardia.