Ohtuvayre™ (ensifentrine) is the first inhaled therapy for the maintenance treatment of chronic obstructive pulmonary disease (COPD) in adult patients, combining both bronchodilator and non-steroidal anti-inflammatory properties within a single molecule.
Developed by Verona Pharma, a biopharmaceutical company based in the UK, Ohtuvayre stands out as a first-in-class selective dual inhibitor of the enzymes phosphodiesterase 3 (PDE3) and phosphodiesterase 4 (PDE4).
The US Food and Drug Administration (FDA) approved Ohtuvayre™ for the maintenance treatment of COPD in June 2024, based on the Phase III clinical programme ENHANCE, following the acceptance of the new drug application for review in August 2023.
Nuance Pharma, a biopharmaceutical company based in China, secured an exclusive agreement with Verona Pharma to develop and market ensifentrine in Greater China in 2021. The drug is being evaluated in China under the ENHANCE-China Phase III clinical trial.
Ohtuvayre’s administration
Ohtuvayre is available as a sterile, yellow to pale yellow aqueous inhalation suspension with a dose strength of 3mg/2.5ml (1.2mg/ml) in low-density polyethylene unit-dose ampules for oral inhalation.
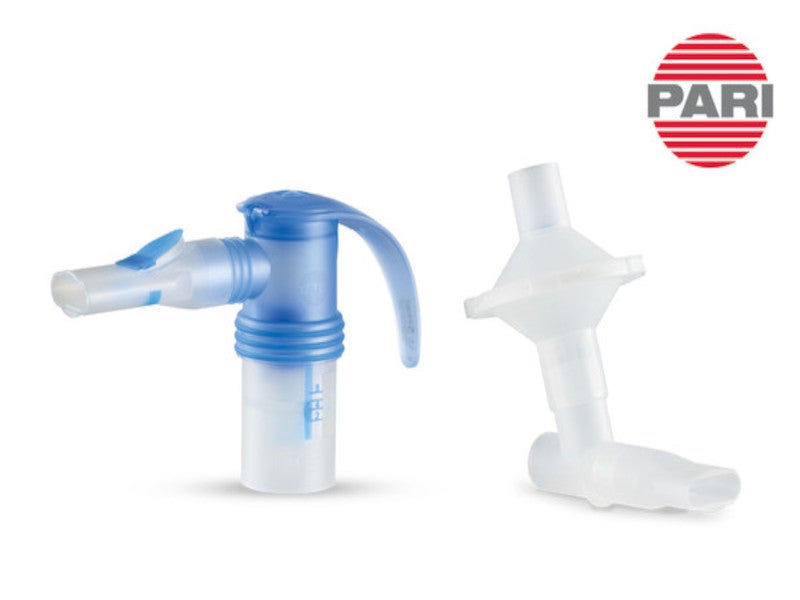
It is administered directly to the lungs via a standard jet nebuliser with a mouthpiece, simplifying the process for patients by eliminating the need for high inspiratory flow rates or intricate hand-breath coordination.
Chronic obstructive pulmonary disease (COPD) causes and symptoms
COPD is a prevalent lung condition characterised by impeded airflow and respiratory difficulties, often referred to as emphysema or chronic bronchitis.
Primary causes of COPD include smoking and exposure to air pollution. Other contributing factors are childhood asthma and a rare genetic disorder known as alpha-1 antitrypsin deficiency, which can cause early-onset COPD.
Symptoms of the condition include persistent coughing, sometimes with phlegm, shortness of breath, wheezing, fatigue and a chronic cough. COPD patients also face an elevated risk of developing other health complications, including lung infections, lung cancer, cardiovascular issues, muscle weakness, osteoporosis, depression and anxiety.
There is no cure for COPD, but symptom management is possible through smoking cessation, avoiding air pollutants, vaccination against infections and treatments involving medications, oxygen therapy and pulmonary rehabilitation.
Ohtuvayre’s mechanism of action
Ensifentrine is a small molecule that acts as an inhibitor of PDE3 and PDE4 enzymes. PDE3 breaks down cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) messenger molecules, while PDE4, located in lung cells, specifically targets cAMP.
The inhibition of the phosphodiesterase enzymes prevents the breakdown of the messenger molecules, which leads to increased levels of cAMP and cGMP in smooth muscle cells, promoting relaxation of bronchial muscles and causing blood vessels to dilate. It also reduces pro-inflammatory mediators.
The accumulation of cAMP and cGMP triggers a series of downstream signalling effects that contribute to improved lung function.
Clinical trials of Ohtuvayre
The ENHANCE clinical programme, encompassing ENHANCE-1 and ENHANCE-2 clinical trials, was pivotal in the FDA approval of Ohtuvayre.
Both studies were designed as 24-week randomised, double-blind, placebo-controlled, parallel-group clinical trials, collectively enrolling 1,553 adults with moderate to severe COPD to evaluate the efficacy of Ohtuvayre.
ENHANCE-1 involved 763 patients who were randomised in a 5:3 ratio to receive either a 3mg dose of Ohtuvayre™ or a placebo, administered through nebuliser devices developer PARI’s LC Sprint® standard jet nebuliser.
ENHANCE-2 included 790 patients, also randomised in a 5:3 ratio, to receive the same dosage of Ohtuvayre via the same administration method twice daily or placebo.
The primary endpoint for both ENHANCE-1 and ENHANCE-2 was the change from baseline in FEV1 AUC0-12h post-dose at week 12. FEV1 – forced expiratory volume in one second – measures the amount of air a person can forcefully exhale in one second, which is a key indicator of lung function, and AUC0-12h represents the area under the curve for FEV1 over 12 hours post-dose.
Improved FEV1 values correlate with better breathing ability, reduced symptoms and overall improved quality of life for patients.
In ENHANCE-1, the mean increase in FEV1 was 61ml in the Ohtuvayre group compared to -26ml in the placebo group. In ENHANCE-2, the mean increase in FEV1 was 48ml in the Ohtuvayre group compared to -46ml in the placebo group.
Ohtuvayre achieved the primary endpoint in both trials, showing a statistically significant and clinically meaningful improvement in lung function compared to placebo.
The St George’s Respiratory Questionnaire (SGRQ), a disease-specific tool, was used to evaluate the impact on overall health, daily life and perceived well-being in patients in both trials. In ENHANCE-1, the SGRQ responder rate for Ohtuvayre at week 24 was 58.2% versus 45.9% for placebo. ENHANCE-2 reported an SGRQ responder rate of 45.4% for Ohtuvayre™ at week 24, compared to 50.3% for placebo.
In the ENHANCE trials, Ohtuvayre™ demonstrated significant clinical benefits, both as a standalone treatment and when used alongside other maintenance therapies, exhibiting a favourable tolerance profile across a diverse group of moderate to severe COPD subjects.
The most common adverse reactions were back pain, hypertension, urinary tract infections and diarrhoea.
Further development is underway for a fixed-dose combination of ensifentrine with glycopyrrolate, a long-acting muscarinic antagonist for COPD maintenance treatment.
Ensifentrine also shows potential for application in the treatment of non-cystic fibrosis bronchiectasis, cystic fibrosis, asthma and other respiratory diseases.