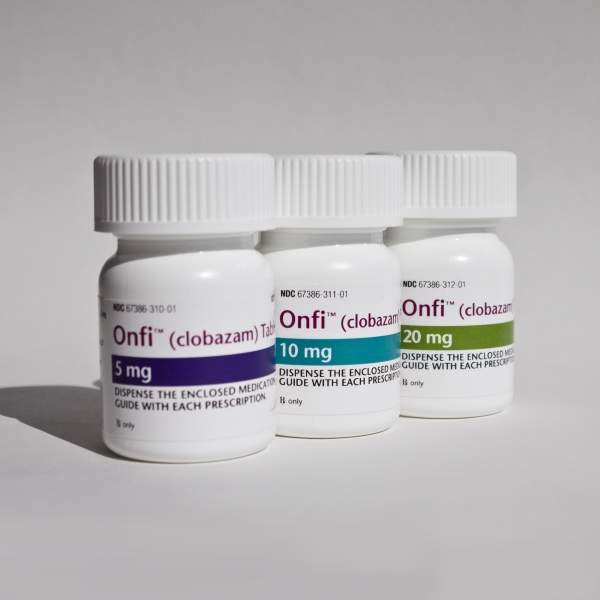
Onfi (clobazam) is an antiepileptic drug approved to treat seizures that are associated with Lennox-Gastaut syndrome (LGS) in patients who are aged two years and above. The drug has been developed by Lundbeck.
The US FDA (Food and Drug Administration) approved Onfi in October 2011 as an adjunctive treatment for patients with LGS.
Lennox-Gastaut syndrome (LGS) details
Lennox-Gastaut syndrome (LGS) is a rare orphan epilepsy disorder that usually begins in children before they reach four years of age.
The disease is diagnosed generally between the second and sixth year of life. It is characterised by regular seizures and multiple seizure types.
It is estimated that more than 50 million people are affected by epilepsy worldwide and more than three million in the US alone. It is also estimated that LGS is responsible for one to four percent of all childhood epilepsies and 80% of the patients with LGS are expected to have continued seizures throughout childhood and into their adulthood.
Onfi mechanism of action
Onfi is an antiepileptic drug that can be administered orally. It is available in 5mg, 10mg and 20mg tablet forms. The precise mechanism of action of the drug is still not clear but it is understood that the drug which contains 1,5 benzodiazepine treats seizures by enhancing the effect of the neurotransmitter gamma-aminobutyric acid (GABA).
Clinical drug trials for clobazam
Phase I clinical trials on Onfi showed the efficacy and tolerability of the drug. Lundbeck conducted Phase II clinical trials on the drug between October 2005 and October 2006.
It was a randomised and double-blind study which evaluated the safety and efficacy of the drug in treating seizures in LGS patients aged between two and 30 years of age. The trials were conducted on 68 patients across ten study centres in the US. The primary outcome measure of the study was finding the reduction in number of drop seizures in 15 weeks time.
The secondary outcome measures included parent / caregiver global evaluations and an overall reduction in seizure rates.
FDA approval for Onfi was based on the Phase III clinical trial, named CONTAIN. The study was conducted between August 2007 and April 2010. It was a double-blind, placebo-controlled study on patients with LGS.
The multicentred study was conducted on more than 238 patients aged between two and 60 years of age. The primary endpoint of the study was the reduction in atonic, tonic or myoclonic seizures from the four-week baseline period compared to the 12-week maintenance period.
The study results found Onfi safe and effective in reducing the average weekly rate of seizures in more than 50% of the patients out of 77.6% who received the high-dose of the drug. The common adverse reactions found included tiredness or sleepiness, drooling, fever, aggressive behaviour, irritability, constipation and lack of coordination. Less than one percent of patients have discontinued the clinical trial due to the adverse reactions.
Lundbeck initiated a Phase III clinical trial on Onfi in December 2005 which is scheduled to be completed in the first quarter of 2012. It is a multicentred, open-label study being conducted on 217 LGS patients who are aged between two and 60 years old.
The interim results of the study were presented in October 2011, which showed that the drug was effective in reducing the average weekly rate of drop seizures by 71.1% in a month. The maximum dose administered in study was 80mg a day.
Marketing commentary for Lundbeck’s LGS treatment drug
Lundbeck has launched the drug across the US market in January 2012. Lundbeck plans to replace Onfi with antidepressant escitalopram drugs, such as Lexapro in the US and Cipralex in other markets, whose patents will expire in 2012 and 2014 respectively. Lundbeck plans to achieve $100m in Onfi sales by 2016.