Opdualag™ is a first-in-class, fixed-dose dual immunotherapy combining nivolumab and relatlimab, approved for the treatment of unresectable or metastatic melanoma in patients aged 12 years and older.
Developed by US-based pharmaceutical company Bristol-Myers Squibb (BMS), Opdualag is available as a single-dose vial containing clear to opalescent, colourless to slightly yellow injectable solution containing 240mg nivolumab and 80mg relatlimab per 20ml (12mg and 4mg per ml) for intravenous administration.
Regulatory approvals for Opdualag
In September 2021, BMS was awarded priority review status by the US Food and Drug Administration (FDA) for the biologics license application (BLA) of the fixed-dose combination of relatlimab and nivolumab (Opdivo).
Opdualag was approved by the FDA for the treatment of adult and paediatric patients aged 12 years and above and weighing at least 40kg with unresectable or metastatic melanoma, in March 2022.
In July 2022, the company received a positive opinion from the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) recommending the approval of Opdualag in Europe for the condition. The European Commission (EC) approved the drug for the first-line treatment of advanced melanoma in adults and adolescents aged 12 years and older with tumour cell PD-L1 expression less than 1%, in September 2022.
Causes and symptoms of metastatic melanoma
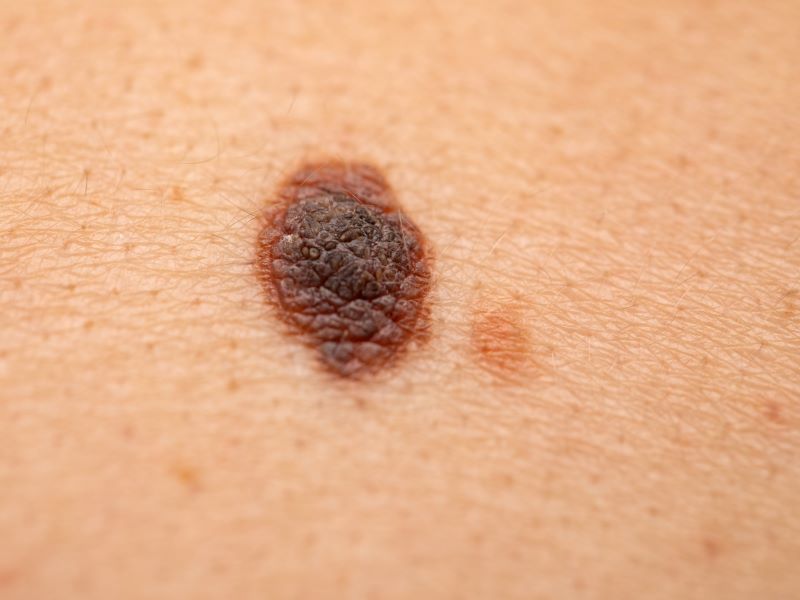
Melanoma is a type of skin cancer that occurs when pigment-producing cells, called melanocytes, become cancerous.
Metastatic melanoma occurs when the cancer spreads to other sites of the body. Approximately 7,990 deaths from melanoma will occur in the US in 2023, according to estimates by the American Society of Clinical Oncology (ASCO). The survival rate of the disease decreases with detection at later stages.
Signs and symptoms of metastatic melanoma may include fatigue, weight loss, swollen or painful lymph nodes, loss of appetite, trouble breathing or coughing, bone pain, headaches, seizures, and swelling of the liver.
Major risk factors for melanoma include frequent exposure to sun or ultraviolet (UV) radiation, a family history of melanoma, and certain genetic mutations.
Exposure to environmental factors such as radiation or vinyl chloride, multiple blistering sunburns, having large numbers of moles, especially atypical moles, and having light skin are some other risk factors.
Opdualag’s mechanism of action
Nivolumab and relatlimab are human immunoglobulin G4 (IgG4) monoclonal antibodies and their combination has been shown to increase T-cell activation in comparison to monotherapy treatment.
Relatlimab is a lymphocyte activation gene-3 (LAG-3) blocking antibody that binds to the LAG-3 receptor and reduces the inhibition of immune response that occurs through the LAG-3 pathway.
Nivolumab is a programmed death receptor-1 (PD-1) blocking antibody that binds to the PD-1 receptor and blocks its interaction with the PD-L1 and PD-L2 ligands.
It reduces the inhibition of an immune response and generates an anti-tumour response.
LAG-3 and PD-1 are distinct immune checkpoint pathways that may potentially act synergistically to produce anti-tumour immune activity, thereby inhibiting tumour growth and promoting tumour regression.
Clinical trials on Opdualag
The approval of Opdualag was based on data from the global, randomised, double-blind Phase II/III RELATIVITY-047 clinical trial, which evaluated the efficacy and safety of the drug in 714 patients with previously untreated metastatic or unresectable Stage III or IV melanoma.
The study compared nivolumab and relatlimab fixed-dose combination against nivolumab alone in patients with previously untreated metastatic or unresectable melanoma.
Patients were randomised to receive Opdualag (nivolumab 480mg and relatlimab 160mg) or nivolumab 480mg every four weeks until disease progression, unacceptable toxicity or withdrawal of consent. The primary endpoint was progression-free survival (PFS) as assessed by Blinded Independent Central Review (BICR) using Response Evaluation Criteria in Solid Tumours (RECIST v1.1).
The results of the study showed that a statistically significant improvement in PFS was achieved in the Opdualag arm compared with the nivolumab arm.
The median PFS was 10.1 months in the Opdualag arm and 4.6 months in the nivolumab arm.
The final analysis of overall survival (OS), the secondary endpoint, was not statistically significant as the median OS was not reached (NR) in the Opdualag arm against 34.1 months in the nivolumab arm.
The most common adverse reactions reported in the Opdualag arm were musculoskeletal pain, fatigue, rash, pruritus, and diarrhoea.
The approval of Opdualag in the European Union was based on an exploratory analysis of the RELATIVITY-047 data in patients with tumour cell PD-L1 expression of less than 1%.
The drug more than doubled the median PFS compared to nivolumab monotherapy. Median PFS in patients receiving Opdualag was 6.7 months, compared to 3.0 months in those receiving nivolumab monotherapy.
No new safety events were identified with the combination.