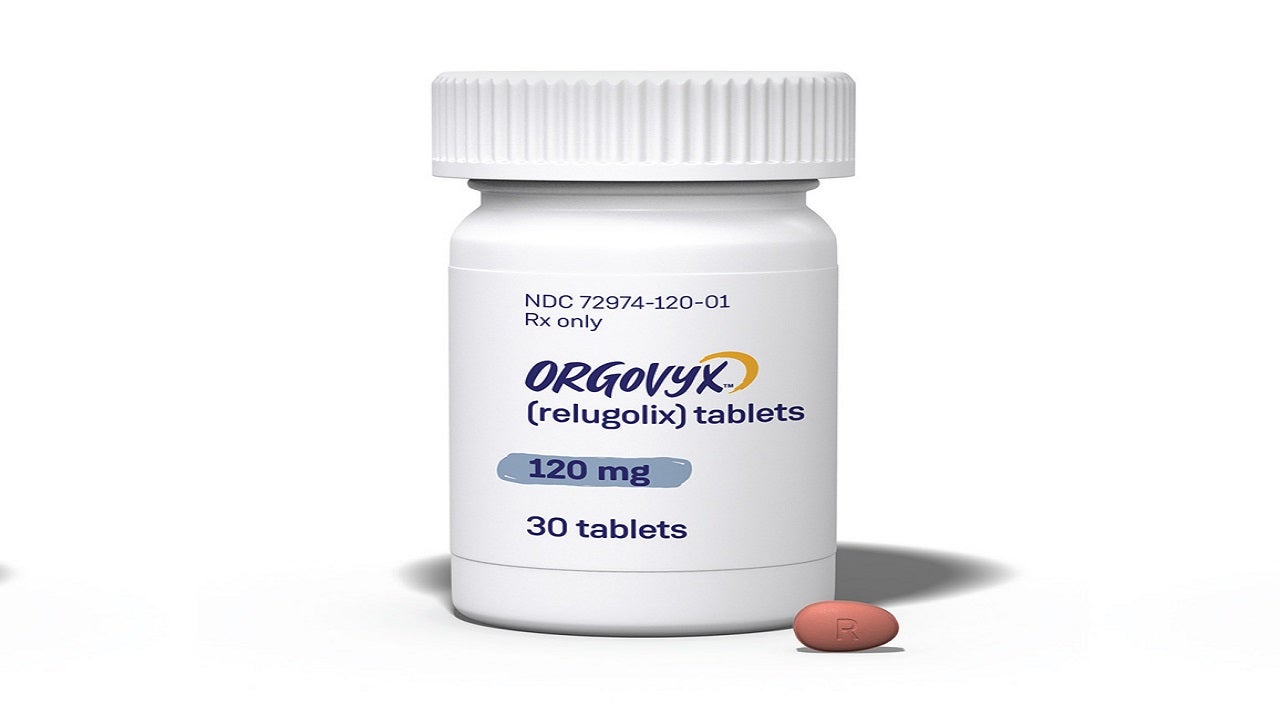
ORGOVYX™ (relugolix) is the first and only oral gonadotropin-releasing hormone (GnRH) receptor antagonist indicated for the treatment of advanced prostate cancer in adult patients.
Developed by Myovant Sciences, ORGOVYX is available through authorised speciality distributors as light red, almond-shaped, film-coated tablets in 120mg dosage strength for oral administration.
ORGOVYX approvals
A new drug application (NDA) for ORGOVYX was submitted to the US Food and Drug Administration (FDA) in April 2020, which was accepted for priority review in June 2020.
Myovant Sciences also submitted a separate NDA to FDA for oral relugolix combination tablet (relugolix 40mg, estradiol 1.0mg, and norethindrone acetate 0.5mg) for women with heavy menstrual bleeding associated with uterine fibroids in May 2020. The application is under review and regulatory decision is expected in June 2021.
The FDA approved ORGOVYX for the treatment of adults with advanced prostate cancer in December 2020.
Another NDA submission for relugolix combination tablet for women with endometriosis is projected in the first half of 2021.
Myovant collaborated with Pfizer to co-develop and commercialise ORGOVYX (relugolix) in oncology and women’s health in the US and Canada. Pfizer will also have an exclusive option to market relugolix in oncology outside the US and Canada, excluding certain Asian countries.
Prostate cancer causes and symptoms
Prostate cancer is a common type of cancer that begins in the prostate gland and typically progresses slowly and mostly remains confined, or localised, to the prostate gland.
Cancer cells may expand outside the prostate gland and spread to surrounding tissues, often called metastasis. Most prostate tumours are diagnosed when men are asymptomatic.
Prostate cancer is the second most prevalent cancer in the world and the fifth major cause of cancer-related death.
It develops through four stages in which the stage one prostate cancer remains confined or localised to the prostate, while stage four prostate cancers are metastatic and spread beyond the prostate. The diagnosis of prostate cancer is dependent on a prostate biopsy.
Symptoms of metastatic prostate cancer can include loss of weight, pain in the bone, tiredness and urinary symptoms.
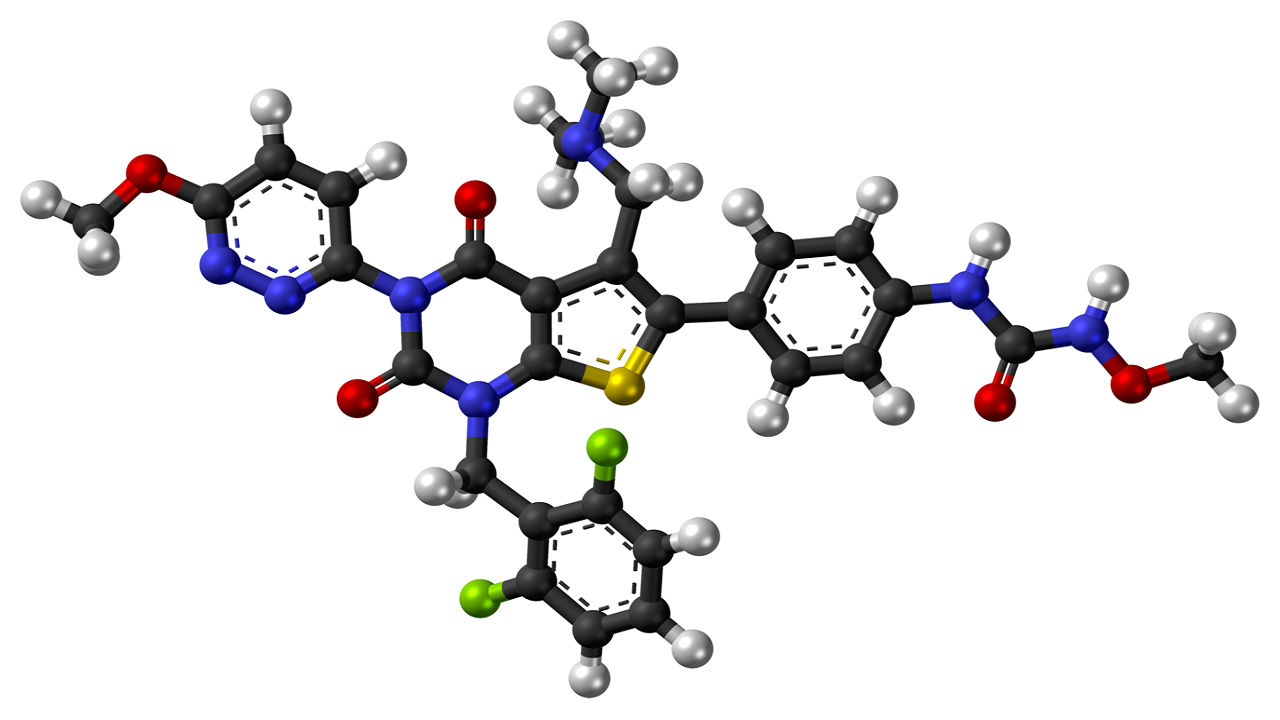
Relugolix mechanism of action
Relugolix is a small nonpeptide molecule that binds competitively to pituitary GnRH receptors, reducing the release of luteinising hormone (LH) and follicle-stimulating hormone (FSH) and, subsequently, testicular testosterone, a hormone considered to trigger the development of prostate cancer.
It is not clear if ORGOVYX is safe or effective in women or children.
Clinical trials on ORGOVYX
FDA approval of ORGOVYX was based on the randomised, open-label, phase three clinical study, HERO, in men with advanced prostate cancer requiring at least one year of androgen deprivation therapy.
A total of 934 recruited patients were randomised to receive ORGOVYX (loading dose of 360mg on the first day followed by daily doses of 120mg) or LEUPROLIDE ACETATE (22.5mg injection subcutaneously every three months) in a 2:1 ratio for 48 weeks.
LEUPROLIDE ACETATE 11.25mg dosage regimen was used in Japan and Taiwan but not recommended in the US for the indication.
The medical castration rate was measured as the primary endpoint of the study.
ORGOVYX achieved a sustained suppression of testosterone to castrate levels (<50ng/dl) over 48 weeks in 96.7% of men compared to 88.8% of men receiving LEUPROLIDE ACETATE injections, the current standard of treatment.
ORGOVYX reduced prostate-specific antigen (PSA) by an average of 65% on day 15, 83% on day 29, 92% after three months and the PSA levels remained suppressed during the treatment period of 48 weeks.
A sub-study was conducted in 137 patients not administered with subsequent androgen deprivation therapy for at least 90 days after ORGOVYX discontinuation. In the study, 55% of men treated with ORGOVYX attained normal testosterone levels (>280ng / dl) or returned to baseline within 90 days of discontinuation of treatment.
The most frequent side-effects of ORGOVYX reported in the patients during the clinical trial include increased glucose and triglycerides, hot flush, decreased haemoglobin, mild to moderate diarrhoea, constipation, fatigue, increased levels of certain liver enzymes and musculoskeletal pain.