ORILISSA (elagolix) is an oral gonadotropin-releasing hormone (GnRH) antagonist indicated for the treatment of moderate-to-severe pain associated with endometriosis.
One of the first drugs to be approved for the condition, ORILISSA was discovered by Neurocrine Biosciences and developed in collaboration with biopharmaceutical company AbbVie under a collaboration and license agreement signed in June 2010.
AbbVie and Neurocrine Biosciences submitted a new drug application (NDA) for ORILISSA™ to the US Food and Drug Administration (FDA) in September 2017. The FDA granted priority review to the drug in October 2017 and approved it in July 2018.
Endometriosis symptoms and causes
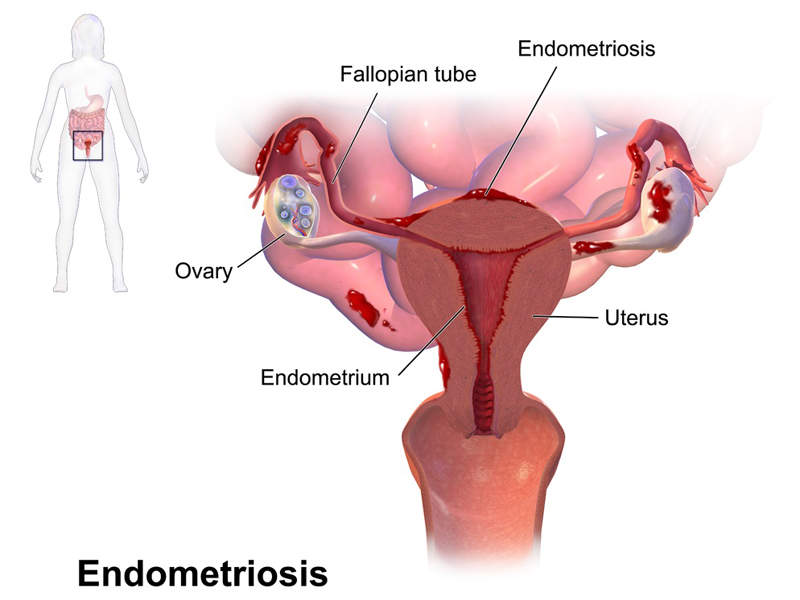
Endometriosis is a painful gynaecological disorder where the endometrium tissue begins to grow outside the uterus. The condition is considered one of the most common causes of infertility.
The disorder affects around 5%-10% of reproductive-age women. Symptoms include chronic pelvic pain during the menstrual period and excessive bleeding and pain with bowel movements or urination.
Treatment options for endometriosis are limited and may involve repeated surgeries or hormonal therapies. The pain management option often used by women suffering from endometriosis include non-steroidal anti-inflammatory drugs or opioids.
ORILISSA’s mechanism of action
ORILISSA™ contains elagolix sodium, which is a non-peptide GnRH receptor antagonist.
The elagolix contained in the drug inhibits GnRH signals by binding to GnRH receptors in the pituitary glands. The drug suppresses the production of luteinising hormones (LH) and follicle-stimulating hormones (FSH) in the pituitary glands, decreasing the level of ovarian sex hormones, estradiol and progesterone in the blood.
The drug is available for oral administration in tablet form in 150mg and 200mg doses.
Clinical trials on ORILISSA
The US FDA’s approval of ORILISSA™ was based on data from two six-month multi-national double-blind, placebo-controlled trials named EM-1 and EM-2.
The clinical trials involved the participation of 1,686 pre-menopausal women with a median age of 32 years.
In total, 952 women were administered with ORILISSA™. Of these, 475 were administered a 150mg once-daily dose, while 477 were administered a 200mg twice-daily dose. The remaining 734 women were treated with placebo.
Both the placebo-controlled trials assessed the reduction of pain associated with endometriosis over the six-month treatment period. The majority of the women that were treated with ORILISSA™ experienced a reduction in pain compared with placebo without any increase in the use of non-steroidal anti-inflammatory drugs or opioids.
The most common adverse events reported in the trials were hot flushes, headaches and nausea. Some of the side effects also included an absence of periods, anxiety, joint pain and depression.
Marketing commentary on AbbVie and Neurocrine Biosciences
Headquartered in North Chicago, Illinois, AbbVie is engaged in the discovery and development of drugs in therapeutic areas such as virology, immunology, oncology, general medicine, and neuroscience.
The company originated as a spin-off of Abbott Laboratories in 2013.
Neurocrine Biosciences is a US-based biopharmaceutical company that develops medicines for neurological and endocrine-based disorders.