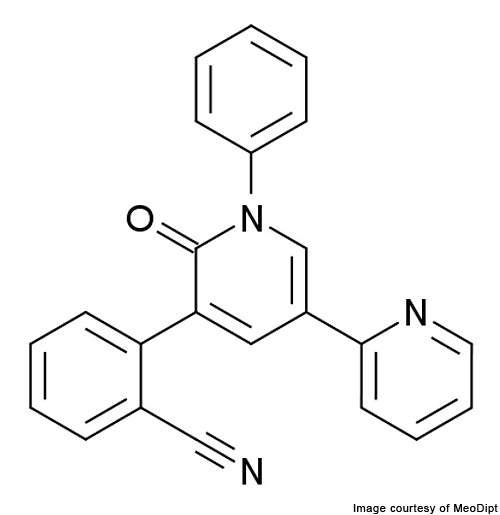
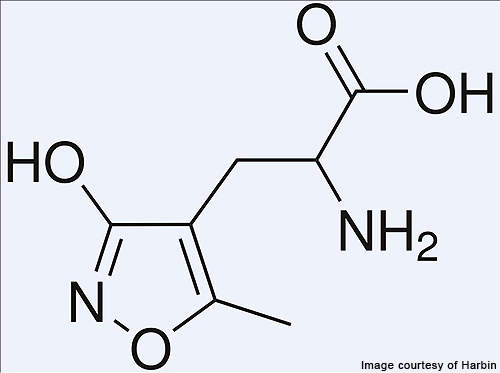
Fycompa (perampanel / E2007) is an AMPA-type glutamate receptor antagonist indicated for the treatment of epilepsy. It was developed by Japan-based healthcare company Eisai.
Eisai submitted a new drug application to the US Food and Drug Administration (FDA) in May 2011, to use Fycompa for the treatment of partial onset seizures in patients with epilepsy. The application was rejected in August 2011 and Eisai was asked to conduct a reanalysis of the data to support the approval. Eisai resubmitted the NDA to the FDA in December 2011. The resubmission was accepted in March 2012.
Fycompa was approved for the treatment of partial onset seizures in epilepsy patients in October 2012 by the FDA.
Eisai also submitted a Marketing Authorisation Application (MAA) to the European Medicines Agency (EMA) in May 2011. The MAA was approved by the EMA in July 2012.
Epilepsy
Epilepsy is a chronic neurological ailment which affects about 45 million people worldwide. It may be hereditary or caused by an illness or brain injury. In 70% of the cases, however, the exact cause is not known. The disease is characterised by frequent seizures caused by abnormal activity in the brain.
Communication between brain cells takes place through electrical signals, which are released in an orderly manner. In epilepsy these electrical signals are sent abnormally causing a surge in electrical activity in the brain.
This activity causes partial or generalised seizures leading to loss of awareness, black outs and convulsions.
There is no cure available for epilepsy. Existing treatment methodologies target just the symptoms of the disease, such as seizures. These treatments are effective in most epilepsy patients but more than 30% patients fail to achieve sufficient seizure control.
Fycompa – AMPA-type glutamate receptor antagonist
Clinical evidence suggests that alpha amino-3-hydroxy-5-mrthyl-4-isoxazolepropionic acid (AMPA) receptors play a key role in several neurodegenerative disorders.
AMPA receptor is a type of receptor for glutamate (a primary molecule in cellular metabolism) that plays a key role in neurotransmission and synaptic transmission.
Excessive activation of AMPA receptors damages brain cells by increasing the flow of calcium into the brain. This condition leads to seizures in patients.
Fycompa works by inhibiting the excess activity of AMPA receptors. Its action reduces the excitatory-inhibitory nerve imbalance caused in the brains of epilepsy patients. Consequently fycompa helps in reducing damage to brain cells and preventing seizures.
Clinical trials
Fycompa was effective in all the five animal seizure models developed in preclinical trials. The drug was initially tested in patients with Parkinson’s disease but failed in phase III clinical trials. In 2008, Eisai discontinued clinical trials of fycompa for Parkinson’s disease.
Three phase II studies were conducted for refractory partial seizures in epilepsy patients. The studies indicated that fycompa was well-tolerated and effective in treating refractory partial seizures.
The drug was investigated in three global phase III clinical trials, studies 304, 305 and 306. A total of 1,480 patients were recruited for the trials. The EMA and FDA approvals for Fycompa were based on the data from the three pivotal phase III clinical studies.
Study 306 was a randomised, double-blind, parallel-group study conducted in Europe and Asia in 706 patients. Positive results from the study were announced in August 2010.
The results indicated that Fycompa was effective in reducing the median seizure frequency in patients.
Results from studies 304 and 305 were presented in April and September 2011, respectively. Results of study 305 demonstrated that 8mg and 12mg doses of Fycompa were effective in reducing the median seizure frequency.
The third phase III clinical trial, study 304, showed that Fycompa was consistent in the treatment of partial-onset seizures.
An open-label, follow-up study called study 207 in patients who have completed phase III trials was completed in August 2012. The study enrolled 138 patients.
Patients who have completed phase II trials were enrolled in a four-year open label extension trial which started in September 2006. The extension trial was completed by August 2012.
Results of the study demonstrated that Fycompa improved seizure control as long-term treatment in the patients.
Marketing commentary
Only 60-70% of epilepsy patients respond to medication. The remaining may not respond to any type of epilepsy drug. Treatment options for these patients include behavioural modification, neurostimulation and diet modification.
Newer drugs to treat epileptic seizures, therefore, have good market potential. The market for epileptic drugs was valued at $3.5bn in 2009 across the US, Japan, France, Germany, Italy, Spain and the UK. Although newer drugs do not offer more benefits than older drugs, they are easier to use and have fewer adverse side effects.