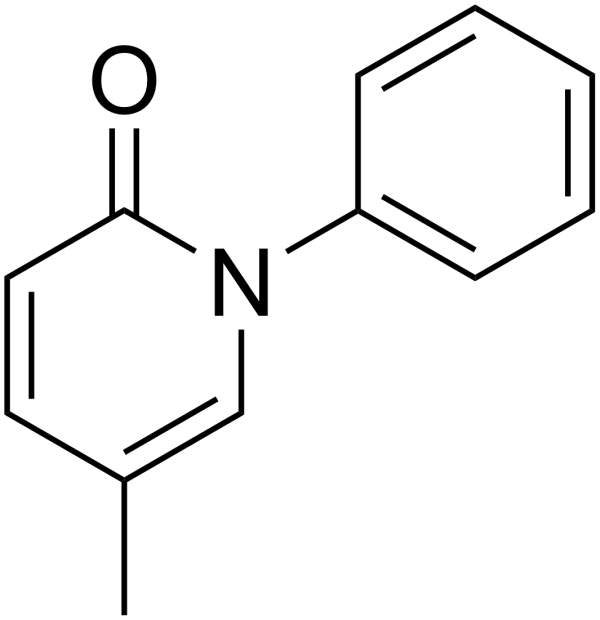
Pirfenidone is a drug indicated for the treatment of Idiopathic Pulmonary Fibrosis (IPF), a disease that causes scarring and thickening of lungs. The drug has been developed by InterMune.
US based Marnac and KDL jointly held the license for the drug earlier. InterMune acquired these two companies in 2007 and gained the rights to market Pirfenidone in US, EU and other countries, except Asia.
The drug’s approval was based on the Phase III trial results released in May 2005. Pirfenidone was approved in Japan for the treatment of IPF patients in October 2008. The drug is being marketed by Shionogi under the trade name Pirespa.
Pirfenidone has been granted orphan drug designation in EU and the US. A new drug application of Pirfenidone was submitted to the US Food and Drug Administration in November 2009. The drug was granted priority review status by the FDA in January 2010.
InterMune received a complete response letter from the FDA, which asked the company to conduct another Phase III trial to evaluate the efficacy of the drug. A Phase III trial was initiated in June 2011.
InterMune submitted a marketing authorisation application for Pirfenidone to the European Medicines Agency in March 2010. The drug was granted approval for the treatment of patients with idiopathic pulmonary fibrosis in March 2011. The drug will be marketed in EU under the trade name Esbriet. It was launched in Germany in September 2011. It will be launched in other EU countries in 2012.
Pirfenidone is also approved in India and is currently being marketed under the trade name Pirfenex by Cipla.
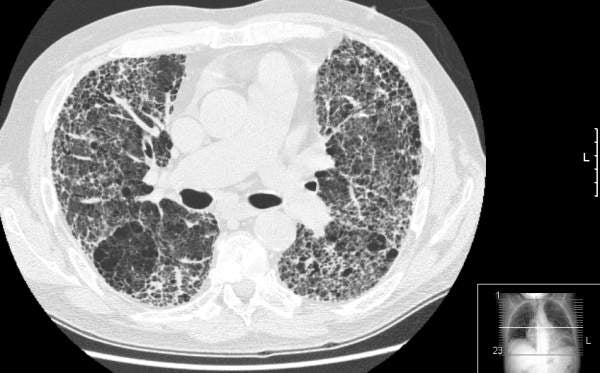
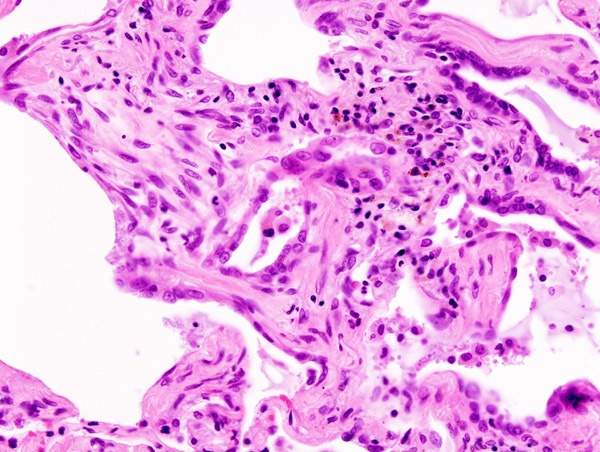
Idiopathic pulmonary fibrosis
The exact cause of IPF is unknown. The disease causes lungs to become scarred and stiffened, which may lead to difficulty in breathing. IPF usually occurs in people aged 50 to 70. It is believed to occur due to inflammatory response from an unknown substance.
IPF may also lead to respiratory failure, pulmonary hypertension, heart failure, pulmonary embolism, pneumonia and lung cancer.
Mechanism of action for Pirfenidone
IPF mainly involves cellular proliferation and enhanced biosynthesis which is caused by cytokine growth factors. The growth factors in the presence of fibroblasts and myofibroblasts may cause excessive formation of collagen and other extracellular matrix thereby causing inflammation.
Pirfenidone has anti-inflammatory and anti-fibrotic properties. It blocks the production and release of pro-inflammatory cytokines and hence decreases the accumulation of inflammatory cells.
Pirfenidone also reduces fibroblast proliferation, production of fibroassociated proteins and cytokines. It reduces excess biosynthesis and accumulation of extracellular matrix, hence reducing the symptoms of IPF.
Clinical trials of the IPF treatment drug
The preclinical studies on IPF were completed by 2004. Five Phase II trials and four Phase III trials are being conducted to evaluate the safety and efficacy of Pirfenidone.
A total of 107 Japanese patients were enrolled for a Phase II trial to evaluate the safety and efficacy of Pirfenidone. The patients were administered either Pirfenidone or a placebo. The results of the trial were released in May 2005. It was observed that Pirfenidone had efficacy parameters.
A Phase II trial was initiated to evaluate and compare the efficacy of Pirfenidone to that of a placebo in IPF patients with Hermansky-Pudlak Syndrome (HPS). It enrolled 58 patients who will be administered either Pirfenidone or sugar placebo pills. The trial which began in March 1997 is expected to be completed by February 2015 with primary completion in September 2010.
A Phase II trial to evaluate the long-term safety and efficacy of the drug in IPF patients was initiated in August 2003. Around 90 patients were enrolled and are being administered around 3,600mg/day of Pirfenidone. The trial is expected to be completed by December 2014 with primary completion in October 2014.
Another Phase II trial was conducted in Japanese patients with IPF to evaluate the safety and efficacy of the drug. The trial was initiated in May 2010 with primary completion in March 2011. Around 50 patients were recruited and administered either Pirfenidone or a placebo.
Phase III development
Phase III trial was conducted in Japanese IPF patients to evaluate the safety and efficacy of the drug. The trial data, released in December 2006, confirmed positive results and the primary end point was met.
CAPACITY-1, a Phase III trial, was initiated in April 2006 to evaluate the safety and efficacy of Pirfenidone. Around 344 patients with idiopathic pulmonary fibrosis were recruited. The patients were administered 2,403mg of pirfenidone a day or were given a placebo. The entire study was completed by November 2008.
A Phase III trial named Capacity- 2 began in June 2006 and was completed by November 2008. The trial was conducted in 435 IPF patients to evaluate the safety and efficacy of the drug. The patients were administered either pirfenidone or a placebo.
The trial data of both the Capacity series of trials were released in February 2009. The primary end point was met. Pirfenidone was found to be safe and well tolerated in patients with IPF.
Another Phase III trial is being conducted in 603 IPF patients. The trial began in August 2008 and is expected to be completed by July 2012. It will evaluate the long-term safety and efficacy in patients who have already participated in Capacity 1 and 2 trials.