Portrazza (necitumumab) is a recombinant human lgG1 monoclonal antibody developed by Eli Lilly, for the treatment of metastatic squamous non-small cell lung cancer (NSCLC).
Eli Lilly received approval from the US Food and Drug Administration (FDA) for Protrazza in combination with gemcitabine and cisplatin for the treatment of metastatic squamous NSCLC in November 2015. The drug was granted orphan designation by the FDA.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion for the marketing approval of Portrazza for the same indication in December 2015.
Metastatic squamous non-small cell lung cancer
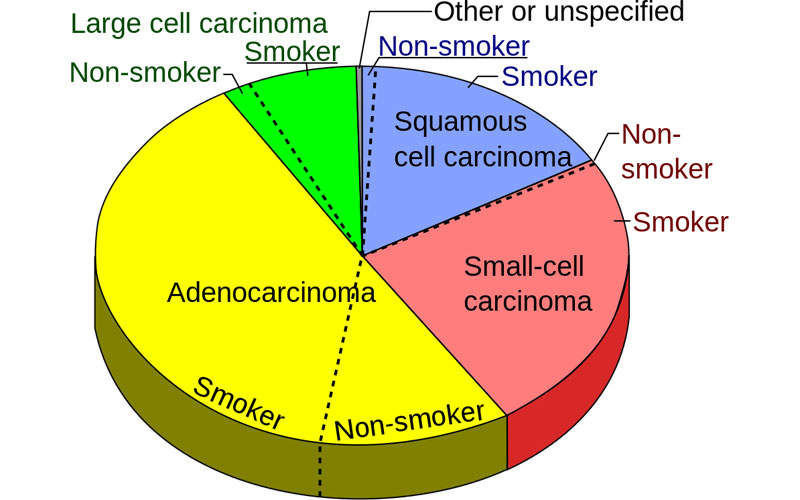
Non-small cell lung cancer is a common type of lung cancer caused by the formation of malignant cells in the lung tissues. NSCLC develops and advances slowly than small cell lung cancer. Smoking is one of the leading causes of non-small cell lung cancer.
NSCLC occurs in three common forms, including squamous cell carcinomas, adenocarcinomas and large cell carcinomas. Early stages of NSCLC do not show any disease symptoms, but in later stages, the patients develop persistent cough, shortness of breath, chest pain, unusual weight loss, loss of appetite and blood in sputum.
Portrazza’s mechanism of action
Protrazza is a recombinant human lgG1 monoclonal antibody that binds to the human epidermal growth factor receptor (EGFR) and stops the binding of EGFR to its ligands. The drug is available in 800mg / 50ml dose vials for intravenous injection.
Clinical trials
The FDA approval for Portrazza is based on the results from a phase III clinical trial known as SQUIRE. It was an open label, multi-centre randomised clinical trial intended to assess the efficacy of first-line treatment with Portrazza in combination with gemcitabine and cisplatin compared to the treatment with gemcitabine and cisplatin in metastatic squamous NSCLC patients.
The study was conducted across 184 investigative sites in 26 countries. It enrolled 1,093 patients with stage IV NSCLC. The results of the study demonstrated that the patients treated with Portrazza showed a significant overall survival with a 16% reduction in risk of death compared to the gemcitabine and cisplatin arm.
The median overall survival in Portrazza arm was 11.5 months compared to 9.9 months in patients treated with gemcitabine and cisplatin alone. The median progression-free survival in Portrazza arm was 5.7 months compared to 5.5 months in the gemcitabine and cisplatin alone arm.
The most common adverse reactions reported during the clinical trial in Protrazza-administered patients were rashes, vomiting, diarrhoea and dermatitis acneiform, while the most severe adverse events reported were venous thromboembolic, pulmonary embolism, cardiopulmonary arrest, hypomagnesemia, coronary artery disease and sudden death.
Marketing commentary
Biologics was selected by Eli Lilly as the speciality pharmacy provider for Portrazza in November 2015.
Other medications available for the treatment of metastatic non-small cell lung cancer include Gilotrif (afatinib) developed by Boehringer Ingelheim Pharmaceuticals and Zykadia (Ceritinib) developed by Novartis Pharmaceuticals.