POSLUMA® (flotufolastat F 18) is a radiohybrid (PET) prostate-specific membrane antigen (PSMA)-targeted positron emission tomography (PET)-imaging agent indicated for PSMA-positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate‐specific antigen PSA level.
Developed by UK-based molecular imaging company Blue Earth Diagnostics, POSLUMA is available as a clear, colourless solution in 8mCi/ml to 158mCi/ml strength for intravenous injection, in a multiple-dose vial.
The US Food and Drug Administration (FDA) approved POSLUMA injection in May 2023. POSLUMA is distributed through 36 radiopharmacies within the national network of PETNET Solutions, the commercial manufacturer and distributor for Blue Earth Diagnostics in the US.
POSLUMA was included in National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines) Prostate Cancer version 2.2023 in July 2023.
Prostate cancer causes and symptoms
Prostate cancer is one of the most common types of cancer that originates in the prostate gland. This sits just below the bladder in males and surrounds the top part of the tube that drains urine from the bladder, called the urethra.
Prostate cancer may remain without any symptoms in its early stages but show signs and symptoms in a more advanced form.
The symptoms include trouble urinating, decreased force in the stream of urine, blood in the urine, blood in the semen, bone pain, losing weight without trying and erectile dysfunction.
POSLUMA’s mechanism of action
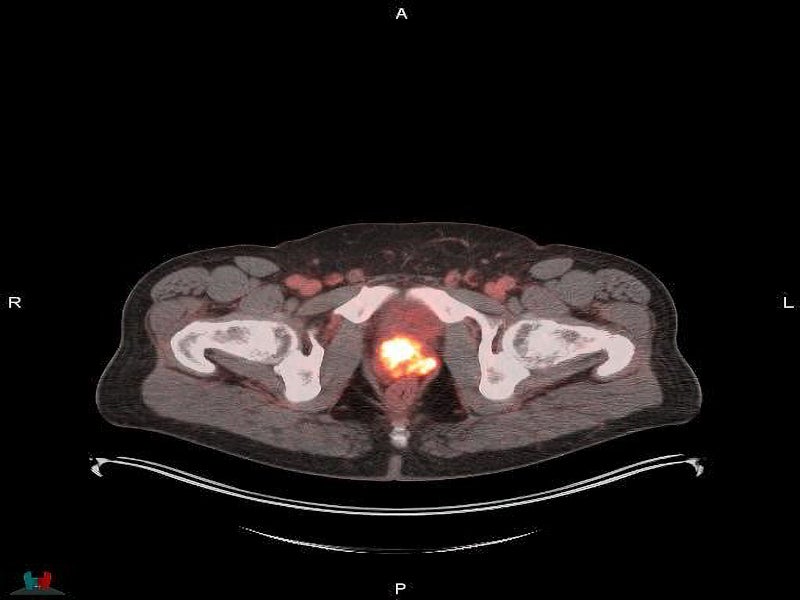
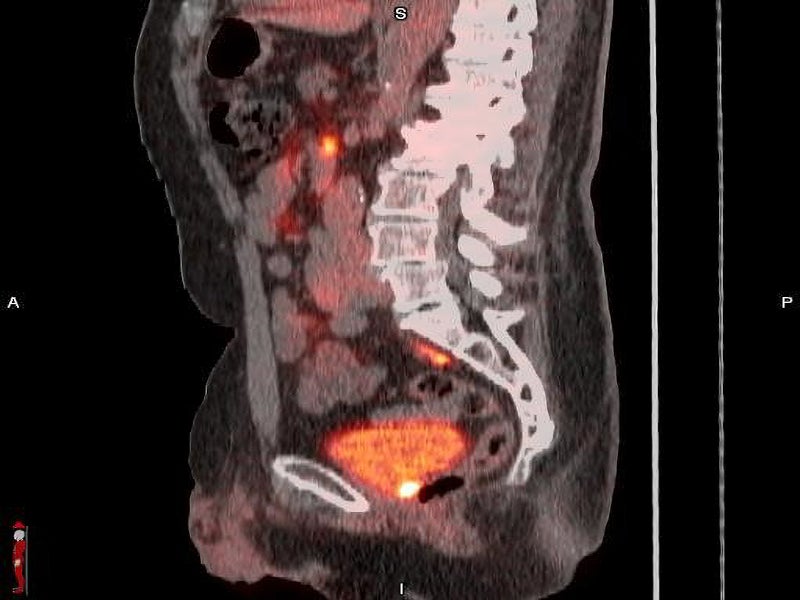
POSLUMA is a radioactive diagnostic agent developed with proprietary radiohybrid (rh) technology. It is designed to bind to PSMA-expressing cells, such as prostate cancer cells, and is then taken up internally.
It is an optimised PSMA-targeted molecule marked with the radioactive fluorine-18 (18F), a beta (ß)+ emitting radionuclide, to be detected using PET. It enables imaging of the prostate and other parts of the body where prostate cancer may have progressed.
Clinical trials of POSLUMA
The safety and diagnostic performance of POSLUMA in 747 patients with initial or recurrent prostate cancer care were evaluated in two prospective, multi-centre, open-label, single-arm Phase Ⅲ clinical trials known as LIGHTHOUSE and SPOTLIGHT, which served as the basis for the FDA approval of POSLUMA.
The LIGHTHOUSE trial enrolled 356 patients diagnosed with unfavourable intermediate-risk or high to very high-risk prostate cancer, who were candidates for radical prostatectomy and pelvic lymph node dissection.
Patients received a single dose of POSLUMA with an administered radioactivity of 8.3 ± 0.62mCi followed by a PET scan from mid-thigh to the base of the skull.
Results from the LIGHTHOUSE trial showed high specificity for the identification of pelvic lymph nodes compared to the histopathological standard of truth in men with PSMA-positive lesions before radical prostatectomy.
The SPOTLIGHT trial enrolled 391 patients with suspected recurrent prostate cancer. All patients received a single dose of POSLUMA with an administered radioactivity of 8.27 ±0.61mCi, followed by a PET scan from mid-thigh to the base of the skull. The results showed high detection rates even at low PSA levels.
The most common side effects reported in patients during the clinical trials were diarrhoea, high blood pressure and soreness at the injection site.