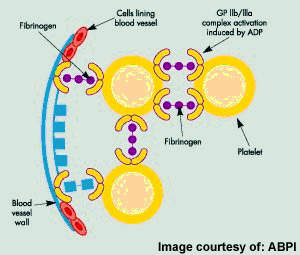
Originally discovered by Sankyo and Ube Industries, prasugrel is now the product of a co-development programme between Daiichi Sankyo and Lilly. The oral antiplatelet drug works by inhibiting platelet activation and subsequent aggregation by blocking the P2Y12 adenosine diphosphate (ADP) receptor on the surface of platelets.
The Phase III clinical trials in the US on the drug for treating patients with ACS undergoing PCI were completed in July 2007, and Daiichi submitted a new drug application to the US Food and Drug Administration (FDA) in December of the same year.
In July 2009, the drug received the FDA approval for treating acute coronary syndrome (ACS) patients undergoing percutaneous coronary intervention (PCI). The European Commission granted marketing approvals for the drug in February 2009.
The drug is currently undergoing Phase II clinical trials in Japan.
Reducing risk of atherothrombosis
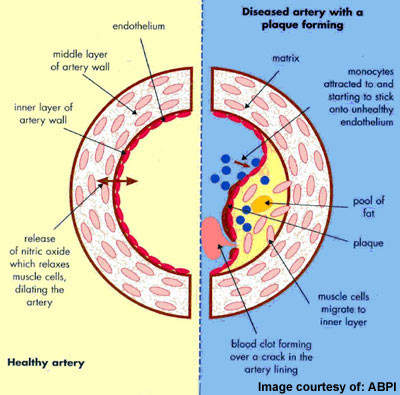
ACS describes a spectrum of disorders, including acute myocardial infarction (heart attack) and unstable angina, which arise from myocardial ischaemia, a reduction in the supply of oxygen to the heart. Acute cardiovascular events, such as myocardial ischaemia, occur when platelets are activated at the site of atherosclerotic plaque rupture and then release substances that lead to platelet aggregation and blood clot formation (thrombosis).
Since most individuals with ACS have atherosclerosis, the underlying cause of heart disease, they are at continued risk of further cardiac events.
Antiplatelet drugs help to reduce the incidence of acute thrombus formation. Thus they are an important component of preventive medical therapy to reduce the risk of further occlusive vascular events in at-risk patients.
Prasugrel goes head-to-head in TRITON TIMI-38 trial
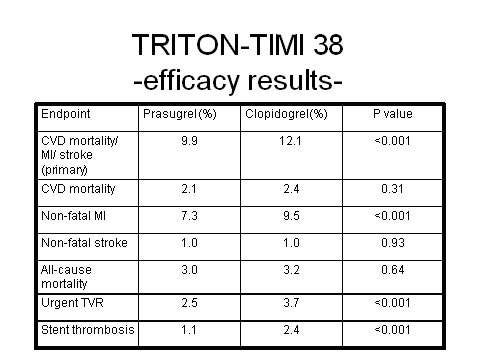
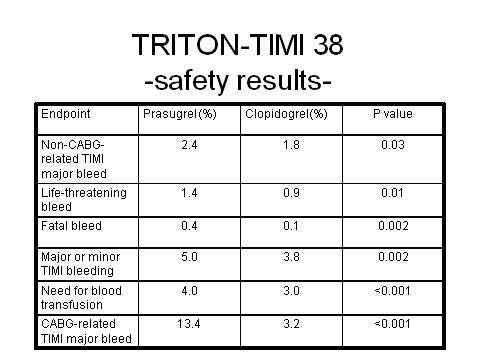
Prasugrel has emerged as a highly potent antiplatelet agent in the Trials to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI 38) trial, in which it was directly compared with fellow antiplatelet clopidogrel.
In this large Phase III trial, a highly significant 19% reduction in relative risk (p=0.0004) for the composite endpoint of cardiovascular death, non-fatal heart attack, or non-fatal stroke was observed for prasugrel plus aspirin in comparison with clopidogrel plus aspirin in the 13,608 ACS patients scheduled for PCI.
Over the 15-month period in which patients were followed, 9.9% of prasugrel-treated patients died from cardiovascular causes, heart attack or stroke, compared with 12.1% of clopidogrel recipients. There was no difference in overall mortality in the two treatment arms.
Overall the trial demonstrated that for every 1,000 patients treated with prasugrel as compared with clopidogrel, 23 myocardial ischaemias were prevented. However, increased efficacy with prasugrel was achieved at the cost of significantly increased major bleeding, life-threatening bleeding and fatal bleeding. Among patients with a history of stroke or transient ischaemic attack, there was also an excess of intracranial haemorrhage in the prasugrel treatment arm.
These findings highlight the dilemma of balancing improved efficacy against the risk of increased bleeding.
Phase III clinical trials on the drug started in November 2004 and concluded in July 2007.
Balancing efficacy and safety
When aspirin, a blood-thinning drug, became standard therapy for heart patients in the 1970s, there was an acknowledged trade off between fewer heart attacks, strokes and cardiovascular deaths, and excess bleeding.
Today, the holy grail of antithrombotic therapy remains the same – to find drugs that reduce the risk of cardiovascular events and death in heart patients without the attendant risk of increased serious bleeding and fatal bleeding.
Prasugrel is clearly an extremely potent investigational antiplatelet agent, with potential to significantly reduce the risk of myocardial ischaemia in ACS patients compared with standard therapy.
However, given that this comes with a significantly increased risk of serious bleeding, the challenge will be to see if certain subsets of patients can be identified for whom the benefits might outweigh the risks. It was notable that the risk of bleeding was particularly high in elderly patients (≥75 years), those with low body weight (≤60 kg), and those with a prior history of transient ischaemic attack or stroke.
The TRILOGY ACS trial evaluated the safety and efficacy of prasugrel against clopidogrel in reducing the risk of cardiovascular death, heart attack or stroke, this time in ACS patients who are managed medically without recourse to coronary revascularisation.
Marketing commentary
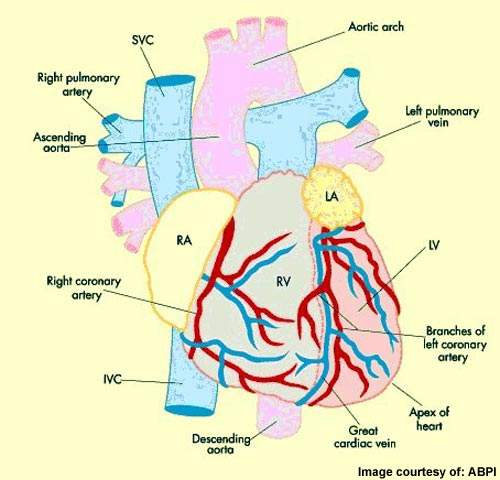
Coronary heart disease, the physical manifestation of atherosclerosis, is a major cause of death and disability. The disease is a significant factor in the 17 million deaths that occur due to cardiovascular disease each year.
Patients with coronary heart disease often have atherosclerosis in other vascular beds, predisposing them to the risk of other occlusive events such as ischaemic stroke.
Antiplatelet drugs, together with antihypertensive and lipid-lowering agents, form part of the panoply of drugs that are used in the management of patients who have experienced an episode of ACS.
Analysts had tipped prasugrel as a potential blockbuster. However, its role in the management of ACS patients is now less clear given safety data from TRITON-TIMI 38.