Provenge (sipuleucel-T) is an autologous cellular immunotherapy used intravenously in the form of infusion for the treatment of men with advanced prostate cancer. The drug was developed by the Dendreon Corporation.
In April 2010, Provenge was approved by the US FDA (Food and Drug Administration) for treating men affected with advanced prostate cancer.
Prostate cancer statistics
Prostate cancer starts in the prostate gland in the male reproductive system. In the advanced stage the disease spreads from the prostate gland to other parts of the body.
It causes difficulty and pain in urinating and also results in erectile dysfunction. The cause of the disease is assumed to be hormonal, genetic, dietary and environmental factors.
It is the most prevalent form of cancer in the US after skin cancer, with estimates showing that more than one million people are affected by the disease. It is the second leading cause of mortalities in the US.
According the US National Cancer Institute, estimates are that more than 220,000 new cases of prostate cancer are reported every year and more than 30,000 of them died of the disease.
Provenge stimulates the immmune system
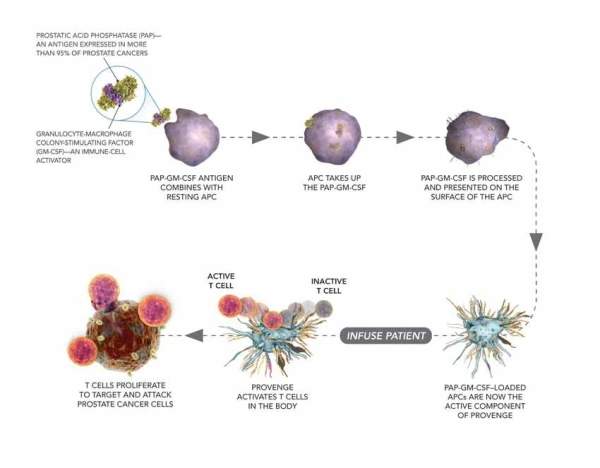
Provenge contains the antigen-presenting cells (APCs) of the patient, which are cultured with a recombinant antigen. The drug works by stimulating the immune system by activating T-cells of the patient, which proliferate and target the prostate cancer cells.
The drug is administered intravenously in a three-dose schedule over approximately one month.
Clinical trials of provenge
Dendreon successfully conducted Phase I clinical trials on provenge. The efficacy and safety of the drug was determined in the trials.
Phase II trials on the drug were conducted between April 2004 and April 2009. It was an open label, non-randomised clinical study that was conducted across 58 locations in the US and Canada.
It enrolled 113 patients with prostate cancer. The primary outcome measure of the study was finding the safety of the drug by administering one infusion of the drug and reviewing the reported adverse events. The secondary outcome measures included evaluation of the efficacy of the drug during a 24-month clinical trial.
Dendreon conducted the first Phase III clinical trials on provenge between November 1999 and September 2004. The study was conducted for 36 months in 34 locations across the US.
It was a double-blind, placebo-controlled study, which was conducted on 127 patients with advanced prostate cancer. The primary outcome measure was finding the time to objective disease progression. The secondary outcome measure included overall survival.
The results of the study showed a statistically significant survival benefit in patients administered with provenge.
The FDA approval for provenge was based on the Phase III clinical trial called IMPACT, which was conducted between July 2003 and January 2009. It was a randomised, double-blind, placebo-controlled and multicentre clinical trial.
The study enrolled 512 male patients above 18 years of age. The primary outcome measure was overall survival. The secondary outcome measure included finding time to objective disease progression.
The results of the study showed an overall increase in survival by 4.1 months. The median survival of the patients who received provenge was 25.8 months, whereas it was 21.7 months in patients who have not received any treatment.
The common adverse reactions reported when administered with provenge included fatigue, chills, fever, headache, back pain, nausea and joint ache.
Marketing commentary
Dendreon plans to introduce provenge in the European market in 2013. Dendreon submitted the marketing authorisation application to the EMA (European Medicines Agency) in early 2012.