Quillivant XR (methylphenidate) is a psychostimulant drug indicated for Attention Deficit Hyperactivity Disorder (ADHD). It was developed by NextWave Pharmaceuticals in collaboration with Tris Pharma.
The US Food and Drug Administration (FDA) approved Quillivant XR for the treatment of ADHD in September 2012.
Attention Deficit Hyperactivity Disorder (ADHD) symptoms and prevalence
ADHD is a psychiatric and neurobehavioral disorder that emerges in children below the age of seven years. Symptoms include inattention, difficulty in being focused and trouble in controlling hyperactivity and behaviour. The disorder affects more boys than girls. It results in restlessness, impulsiveness, and lack of attention which damages the children’s ability to learn.
ADHD is one of most common neurobehavioral conditions diagnosed in the US. According to the Centre for Disease Control Prevention (CDC), an estimated 5.2 million children aged between three and 17 were diagnosed with ADHD in 2010.
Quillivant XR mechanism of action
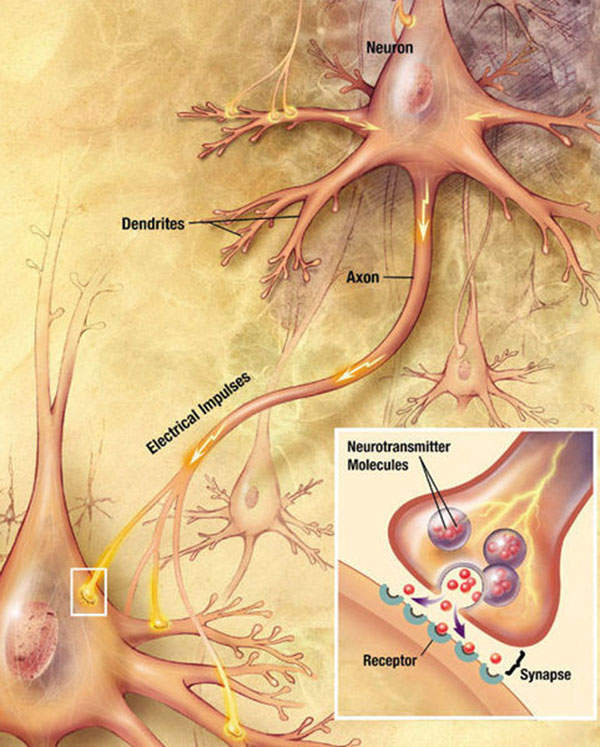
Quillivant XR contains methylphenidate hydrochloride (HCl), a nerve-stimulant drug that increases CNS activity. The drug’s precise mechanism of action is not known, but it is believed to work by blocking the re-uptake of neurotransmitters such as dopamine and norepinephrine. This in turn increases the utility of these neurotransmitters in the brain.
The drug is available in liquid solution for oral administration, and it lasts for 12 hours.
Methylphenidate hydrochloride clinical trials
The FDA approval of Quillivant XR was based on the results of a Phase III clinical trial. It was an open-label, randomised, placebo-controlled, double-blind, multi centre and crossover laboratory classroom study. The trial was conducted by NextWave between May 2009 and August 2009 across two centres in the US. A total of 45 children aged between six and 12 years were enrolled for the study.
The study was conducted for a period of four to six weeks by administering 20mg dose of Quillivant XR once in a day. The drug dose was increased by 10mg or 20mg until the optimisation level of 60mg was reached. After achieving the optimal dose the patients were enrolled into a two-week double-blind crossover treatment. An optimised dose of the drug or placebo was administered during this period. The observers evaluated the attention and behaviour of the patients every week with help of the SKAMP (Swanson, Kotkin, Agler, M-Flynn and Pelham) rating scale.
The study met its primary endpoint. The results of the study demonstrated that Quillivant XR significantly improved ADHD symptoms when compared to placebo. The common adverse reactions found during the study included vomiting, decreased appetite, trouble in sleeping, weight loss, stomach pain, and anxiety.
NextWave initiated another Phase III study in July 2012. It is a multicentre, randomised, placebo-controlled, double-blind, and parallel assignment trial. The study enrolled 80 patients aged between six and 12 years across five centres in the US. The primary outcome measure of the study includes finding the average of seven SKAMP scale scores collected at seven time points in a day. The results of the study are expected to be released by December 2012.
Marketing Quillivant XR in the US
NextWave Pharmaceutical is a leading pharmaceutical company based in California, US. It is focused on the development and marketing of products for the treatment of neurological disorders.
NextWave plans to launch Quillivant XR in the market across the pharmacies in the US by January 2013. The drug was developed using patented OralXR delivery platform developed by Tris Pharma. Other approved medications available in the market for similar indications include Ritalin, manufactured by Novartis, and Dexedrine, developed by Amedra Pharmaceuticals.
Approval of Quillivant XR has filled the need for a once-daily treatment for ADHD. The unique liquid delivery system provides an alternative to patients who have difficulty in swallowing capsules.
In October 2012, Pfizer revealed its plan to acquire NextWave for $700m. The deal is expected to be closed by the end of 2012 pending regulatory approvals.