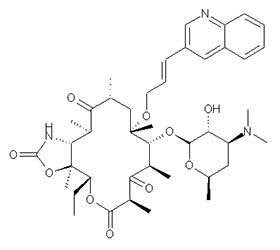
Restanza (cethromycin) is a second-generation ketolide, once-a-day oral antibiotic that is under review for approval by the US Food and Drug Administration (FDA) for the treatment of community-acquired pneumonia (CAP).
In September 2008, Advanced Life Sciences submitted a new drug application for Restanza as a CAP treatment to the FDA. The submission was based on positive results from 53 clinical trials, including two pivotal Phase III trials.
In July 2009, the FDA issued a complete response letter to Advanced Life Sciences, saying that it could not approve the new drug application and that additional clinical trials were needed to prove the efficacy of the drug.
Advanced Life Sciences received further guidance from the FDA on the clinical programme required for the approval of Restanza as a CAP treatment in March 2010. The FDA suggested that a special protocol assessment (SPA) needs to be set up using two clinical trials. According to the FDA, the clinical trials should compare Restanza with an approved macrolide antibiotic. Advanced Life Sciences is currently in the process of finalising the SPA.
Advanced Life Sciences is also developing Restanza as a broad-spectrum medical countermeasure for bio-defence, to combat multiple high-priority bio-terror agents tularaemia, plague and anthrax. The drug is not yet approved for these indications.
Restanza as a CAP treatment
Restanza is Advanced Life Sciences’ lead product candidate and has demonstrated positive Phase III results for the treatment of CAP. The drug has been tested in approximately 5,200 people, and has shown higher in-vitro potency and a broader range of activity against Gram-positive bacteria associated with respiratory tract infections than macrolides.
In in-vitro tests, Restanza also appears effective against bacteria resistant to penicillin, macrolide and fluoroquinolone. Restanza’s potency and ability to overcome bacterial resistance may be due to its mechanism of action, which results in specificity for its bacterial target.
CAP is the sixth most common cause of death in the US. The respiratory tract infection is caused by pathogens such as Streptococcus pneumoniae and Haemophilus influenzae.
The condition affects 5.6 million patients in the US each year, and is particularly common in the elderly. It accounts for 10 million doctor visits and two million hospitalisations each year. Symptoms include cough, fever, difficulty breathing and chest pain.
Efficacy against anthrax infection and plague
Restanza has also demonstrated significant efficacy in preventing anthrax infection post-exposure, as well as treating inhalation anthrax after symptoms of infection have developed.
In September 2009 the FDA granted orphan drug designation to Restanza for the prophylactic treatment of plague and tularaemia. Tularaemia and plague are classified as Category A bio-terrorism agents by the US Centres for Disease Control and Prevention (CDC), which is the highest priority classification. High-priority agents include organisms that pose a risk to national security, can easily be disseminated or transmitted, result in high mortality rates or have the potential to cause a major public health problem.
Positive results were obtained from studies to measure Restanza’s therapeutic efficiency in treating inhalation anthrax after symptoms of infection has developed. The results of the placebo-controlled non-human study showed that a 14-day course of Restanza achieved a 60% survival rate when administered in animals that had already demonstrated clinical symptoms of anthrax infection.
Those receiving a placebo did not survive. Because of the extremely lethal nature of anthrax infection once symptoms appear, Restanza’s ability to achieve a 60% survival rate is clinically and statistically significant.
At the end of September 2009 Advanced Life Sciences announced that results from a non-human primate study involving Restanza demonstrated statistical significance at a 90% survival rate against an inhaled lethal dose of plague.
Following the terrorist attacks of 11 September 2001, the US Government has raised the profile of public health threats from the deliberate use of weapons of mass destruction, chemical, biological, radiological or nuclear agents.
Marketing commentary
Macrolides and penicillin are the front-line treatments for respiratory tract infections such as CAP. As resistance to these treatments grows, there is a need for new antibiotics with unique mechanisms of action that can overcome this emerging resistance.
The estimated total annual cost of healthcare for CAP in the US is $8.4bn with prescribed antibiotics accounting for $2bn annually. The cost of hospitalised CAP patients who do not respond to penicillin or macrolides is $15,000 per patient.